UREA CREAM 40%- urea cream
Urea Cream 40% by
Drug Labeling and Warnings
Urea Cream 40% by is a Prescription medication manufactured, distributed, or labeled by Laser Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- WARNINGS AND PRECAUTIONS
-
DESCRIPTION
Description
Urea 40% is a keratolytic emollient which is a gentle, yet potent, tissue softener for nails and/or skin. Each gram of Urea 40% contains 40% urea as an active ingredient, and the following inactive ingredients: Carbomer, Dimethyl Isosorbide, Emulsifying Wax, Glycerin, Isopropyl Myristate, Neopentyl Glycol Dicaprylate/Dicaprate, Purified Water, Sodium Hydroxide, Tridecyl Stearate, Tridecyl Trimellilate, and Xanthan Gum.
Urea is a diamide of carbonic acid with the following chemical structure:
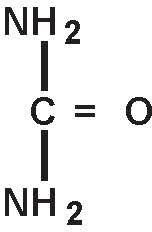
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
-
INDICATIONS & USAGE
Indications and Usage
For debridement and promotion of healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or purulent debris or eshar. Urea is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis pilaris, keratosis palmaris, keratoderma, corns and calluses, as well as damaged, ingrown and devitalized nails.
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
-
PREGNANCY
PREGNANACY
Pregnancy Category B. Animal reproduction studies have revealed no evidence of harm to the fetus, however, there are no adequate and well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, Urea 40% should be given to a pregnant woman only if clearly needed.
- NURSING MOTHERS
- ADVERSE REACTIONS
-
DOSAGE & ADMINISTRATION
Dosage and Administration
Apply Urea 40% to affected skin twice a day, or as directed by your physician. Rub in until completely absorbed.
Apply to diseased or damaged nail(s) twice per day, or as directed by a physician.
All prescription substitutions and/or recommendations using this product shall be made subject to state and federal statutes as applicable. Please note: this is not an Orange Book product and has not been subjected to FDA therapeutic equivalency or other equivalency testing. No representation is made as to generic status or bioequivalency. Each person recommending a prescription substitution using this product shall make such recommendations based on each person's professional opinion and knowledge, upon evaluation of the active ingredients, excipients, inactive ingredients and chemical information provided herein.
-
HOW SUPPLIED
How Supplied
Urea 40% Cream 1 oz.(28.35 g): NDC: 16477-340-01; Urea 40% Cream 3 oz.(85 g): NDC: 16477-340-03; Urea 40% Cream 7 oz.(198.4 g): NDC: 16477-340-07
- STORAGE AND HANDLING
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UREA CREAM 40%
urea creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 16477-340 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UREA (UNII: 8W8T17847W) (UREA - UNII:8W8T17847W) UREA 400 mg in 1 g Inactive Ingredients Ingredient Name Strength ISOSORBIDE (UNII: WXR179L51S) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) TRIDECYL STEARATE (UNII: A8OE252M6L) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) XANTHAN GUM (UNII: TTV12P4NEE) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYSORBATE 60 (UNII: CAL22UVI4M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 16477-340-03 85 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/04/2018 2 NDC: 16477-340-07 198.4 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/04/2018 3 NDC: 16477-340-01 28.35 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/04/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/04/2018 Labeler - Laser Pharmaceuticals, LLC (614417132)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
