STERILE WATER- water irrigant
Sterile Water by
Drug Labeling and Warnings
Sterile Water by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use STERILE WATER FOR IRRIGATION safely and effectively. See full prescribing information for STERILE WATER FOR IRRIGATION.
STERILE WATER FOR IRRIGATION
Initial U.S. Approval: 1974INDICATIONS AND USAGE
Sterile Water for Irrigation is indicated for use as an irrigant. (1)
DOSAGE AND ADMINISTRATION
- For Irrigation only. Not for injection or infusion by usual parenteral routes. (2.1, 5.1)
- The volume and/or rate of irrigation depend on the type of the procedure and the capacity or the surface area of the structure to be irrigated. (2.2)
- For preparation and administration instructions see the full prescribing information. (2.1, 2.3)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- None. (4)
WARNINGS AND PRECAUTIONS
- Hypotonicity: Administration by parenteral routes can result in serious adverse reactions. For irrigation use only. (5.1)
- Excessive Water Absorption: May result in hyponatremia, hypoosmolality and fluid overload. Monitor patients for absorption of clinically relevant amounts of fluid during and after the procedure. Additional monitoring is recommended for patients at increased risk of developing complications of fluid overload syndrome (e.g., patients with renal or cardiac impairment) or hyponatremic encephalopathy (e.g., pediatric patients; women, in particular premenopausal women; patients with hypoxemia; and patients with central nervous system disease). Avoid use in procedures that require longer irrigation times or an irrigation fluid pressure that promotes absorption. (5.2, 8.4)
- Use with Electrosurgery/Cautery: Ensure compatibility with equipment used with electrosurgery or cautery. (5.2)
ADVERSE REACTIONS
Most common adverse reactions are: hyponatremia, fluid overload, fluid absorption, electrolyte imbalance. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Baxter Healthcare Corporation at 1-866-888-2472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dosage
2.3 Preparation and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypotonicity
5.2 Excessive Water Absorption
5.3 Use with Electrosurgery/Cautery
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- For irrigation only. Not for intravenous injection.
- Sterile Water for Irrigation is hypotonic with an osmolarity of zero mOsmol/L. It is intended for use as an irrigation fluid and not for intravenous administration or administration by other, parenteral routes (e.g., subcutaneous or intramuscular) [see Warnings and Precautions (5.1)].
- Sterile Water for Irrigation is not potable water and is not intended for oral administration.
- Sterile Water for Irrigation that has been warmed must not be returned to storage.
2.2 Recommended Dosage
The volume and/or rate of irrigation depend on the type of the procedure and the capacity or the surface area of the structure to be irrigated.
2.3 Preparation and Administration Instructions
For single-dose only. Sterile Water for Irrigation is available in a flexible plastic container and a plastic pour bottle. Use the contents of the opened container immediately to minimize the potential for bacterial growth and pyrogen formation. Discard the unused contents of opened containers, as Sterile Water for Irrigation contains no antimicrobial preservative.
Adding Medications to Either the Flexible Plastic Container or the Plastic Pour Bottle
- Use aseptic technique when making additions to Sterile Water for Irrigation.
- Additives may be incompatible with Sterile Water for Irrigation.
- The compatibility of additives with Sterile Water for Irrigation must be assessed. Before introducing additives, check for a possible color change and/or the appearance of precipitates, insoluble complexes, or crystals.
- Before adding a substance or medication, verify that it is soluble and/or stable in water and that the pH range of Sterile Water for Irrigation is appropriate.
- Additives known or determined to be incompatible should not be used.
- Mix thoroughly after additives have been introduced.
- Use immediately.
Administration Instructions for Irrigation by Gravity Using the Flexible Plastic Container
- 1. Use aseptic technique.
- 2. Tear overwrap down side at slit and remove solution container. Visually inspect the container. If the outlet port protector is damaged, detached, or not present, discard container as solution path sterility may be impaired. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Check for minute leaks by squeezing the container firmly. If leaks are found, discard solution as sterility may be impaired.
- 3. Suspend container using hanger hole.
- 4. Remove plastic protector from outlet port at bottom of container.
- 5. Attach irrigation set. Refer to complete directions accompanying set.
Warming
If desired, warm the flexible plastic container in overwrap to near body temperature in a water bath or fluid warmer heated to not more than 45°C.
Microwave heating of irrigation fluids is not recommended.
Administration Using the Plastic Pour Bottle
- 1. Inspect Sterile Water for Irrigation visually for particulate matter and discoloration prior to use. Discard if the fluid is not clear or the seal is broken.
- 2. Use aseptic technique
- 3. Remove the plastic shrink band by tearing along the perforation line and unscrew the bottle cap.
- 4. Prevent contact of the fluid with the external surface of the container (including the thread for the bottle cap of the plastic pour bottle).
Warming
If desired, warm the plastic pour bottle in a fluid warmer to not more than 50°C for a maximum of 60 days. Discard after 60 days of warming.
Microwave heating of irrigation fluids is not recommended.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypotonicity
Sterile Water for Irrigation is hypotonic with an osmolarity of zero mOsmol/L. It is intended for use as irrigation fluid and not for intravenous administration or administration by other, usual parenteral routes, such as intravascular administration in general, subcutaneous administration, or intramuscular administration.
Serious adverse reactions, including cerebral or pulmonary edema, massive hemolysis, and acute renal failure can result from the hypotonicity of Sterile Water for Irrigation [see Warnings and Precautions (5.2)].
5.2 Excessive Water Absorption
Due to the hypotonicity, absorption of Sterile Water for Irrigation can result in serious adverse reactions of hyponatremia, hypoosmolality and fluid overload, resulting in fatality or permanent morbidity. Complications include cerebral edema, encephalopathy, pulmonary edema, massive hemolysis, rhabdomyolysis, renal failure, and hyperkalemia [see Adverse Reactions (6)]. In addition, excessive volume or pressure of the irrigation fluid may also cause undue distension of body cavities and may cause tissue disruption (e.g., tears or perforation). These complications can manifest after irrigation has ended and immediate intervention may be required.
Monitor patients closely for absorption of clinically relevant amounts of fluid during and for an appropriate period after the procedure. If absorption occurs, discontinue Sterile Water for Irrigation.
Additional close monitoring during and/or after the procedure is recommended for patients at increased risk for developing complications related to hypotonicity, such as:
- fluid overload syndrome, including patients with severe renal impairment, impaired cardiac function, or other clinical conditions associated with edematous states.
- hyponatremic encephalopathy, including pediatric patients; women, in particular premenopausal women; patients with hypoxemia; and patients with underlying central nervous system disease.
Avoid use of Sterile Water for Irrigation in patients with wounds where significant absorption may occur, such as procedures that require irrigation over a longer period of time or an irrigation fluid pressure that promotes absorption (e.g., transurethral resection of prostate). Sterile Water for Irrigation should only be used by clinicians familiar with the treatment of possible complications.
-
6 ADVERSE REACTIONS
The following adverse reactions have been identified during post approval use of Sterile Water for Irrigation. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Metabolism and Nutrition disorders: Hyponatremia, fluid overload, fluid absorption, electrolyte imbalance
Nervous System Disorders: Cerebral edema
General Disorders and Administration Site Conditions: Burning sensation (with irrigation of eyes and skin wounds)
Musculoskeletal and Connective Tissue Disorders: Rhabdomyolysis (myoglobinuria)
Renal and Urinary Disorders: Renal failure
Other Adverse reactions which may occur in association with absorption of water for irrigation include:
Blood and Lymphatic System Disorders: Hemolysis (hemoglobinemia, hemoglobinuria)
Metabolism and Nutrition Disorders: Hypervolemia, hypoosmolality, hyperkalemia, acid/base balance disorder
Nervous System Disorders: Encephalopathy (seizure, loss of vision, lethargy, disorientation, irritability, vomiting, nausea, headache)
Cardiac Disorders: Cardiac arrest, cardiac failure, bradycardia, electrocardiogram abnormal
Vascular Disorders: Hypertension, postoperative hypotension
Respiratory, Thoracic and Mediastinal Disorders: Respiratory arrest, respiratory failure, pulmonary edema -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Appropriate administration of Sterile Water for Irrigation is not expected to cause adverse developmental outcomes. There are no available data on Sterile Water for Irrigation use in pregnant women to determine a drug-associated risk of adverse developmental outcomes. Animal reproduction studies have not been conducted with Sterile Water for Irrigation.
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20% respectively.
8.2 Lactation
Risk Summary
Appropriate administration of Sterile Water for Irrigation is not expected to cause harm to a breastfed infant. There are no data on the presence of Sterile Water for Irrigation in either human or animal milk, the effects on the breastfed infant or the effects on milk production. The lack of clinical data during lactation precludes a clear determination of the risk of Sterile Water for Irrigation to a breastfed infant; therefore, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Sterile Water for Irrigation and any potential adverse effects on the breastfed infant from Sterile Water for Irrigation or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Pediatric patients are at higher risk compared to adults for developing encephalopathy as a complication of hyponatremia, if there is excessive absorption of Sterile Water for Irrigation [see Warnings and Precautions (5.2)].
-
10 OVERDOSAGE
Excessive rate, volume, pressure or duration of irrigation with hypotonic fluid can result in excessive fluid absorption and permanent morbidity or death as a result of hyponatremia, hypoosmolality and/or fluid overload. In the event of clinically relevant absorption of Sterile Water for Irrigation, immediate intervention may be required [see Warnings and Precautions (5.2)].
-
11 DESCRIPTION
Sterile Water for Irrigation contains water that is sterilized and packaged for use as an irrigant. No antimicrobial agent or other substance has been added. The pH is 5.5 (5.0 to 7.0). Sterile Water for Irrigation is hypotonic with an osmolarity of zero mOsmol/L.
The flexible plastic container is fabricated from a specially formulated polyvinyl chloride. The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the fluid significantly. Water in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials.
The plastic pour bottle is fabricated from specially formulated polyolefin. The polyolefin is a copolymer of ethylene and propylene. The container requires no vapor barrier.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Sterile Water for Irrigation is supplied in single-dose plastic pour bottles and flexible plastic containers as follows:
- Container
Product Codes
Fill Volume (mL)
NDC
Plastic Pour Bottle
2F7112
250
0338-0004-02
2F7113
500
0338-0004-03
2F7114
1000
0338-0004-04
2F7115
1500
0338-0004-05
Flexible Plastic Container
2B7114
1000
0338-0003-44
2B7116
2000
0338-0003-46
2B7117
3000
0338-0003-47
Storage
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat.
Store at room temperature (25°C): brief exposure up to 40°C does not adversely affect the product.
-
17 PATIENT COUNSELING INFORMATION
Inform patients, caregivers, or home healthcare providers of the following risks of Sterile Water for Irrigation:
- Hypotonicity [see Warnings and Precautions (5.1)]
- Excessive Water Absorption [see Warnings and Precautions (5.2)]
- SPL UNCLASSIFIED SECTION
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
2F7114
NDC: 0338-0004-041000 mL
Baxter Logo
Not for Injection
For Irrigation Only
Sterile Water for
Irrigation USPPrepared by distillation. No antimicrobial agent or
other substance has been added. pH 5.5 (5.0 to 7.0).
Osmolarity 0 mOsmol/L (calc.). Sterile, nonpyrogenic.
Pour Bottle. Warning: This fluid is not isotonic and is
hemolytic. Dosage and Administration: See prescribing
information. Cautions: Warm in oven to not more
than 50ºC for a maximum of 60 days. Discard after
60 days of warming. Do not use unless fluid is clear
and seal is intact. Discard unused portion. Rx only.
Recommended storage: Room temperature (25ºC).
Avoid excessive heat.PL 325 Plastic
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Made in USA07-09-78-332
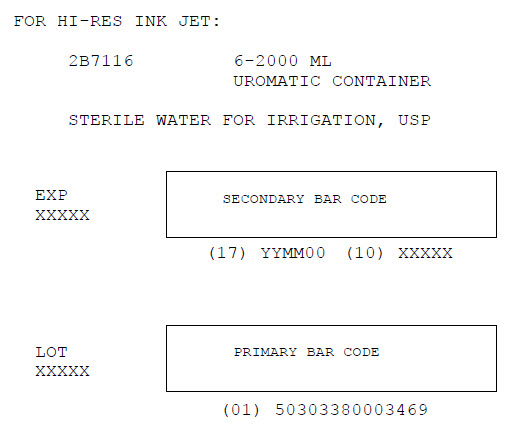
LOT
EXPBar Code Position Only*
0303380004041Bar Code
Sterile Water for
Irrigation, USP
Pour Bottle1000 ML
Qty 12
NDC: 0338-0004-04 2F7114
EXP JUN 08 LOT #G012345Bar Code
(01)50303380004046(17)080630(10)G012345
LOT
EXP
2000 mL
NOT FOR INJECTION
2B7116
NDC: 0338-0003-46
STERILE WATER
For Irrigation USPFor irrigation only Prepared by distillation
No antimicrobial agent or other substance has been added
pH 5.5 (5.0 TO 7.0). Osmolarity 0 mOsmol/L (calc) Sterile
Nonpyrogenic Single dose containerDo not use unless fluid is clear Discard unused portion
Cautions This fluid is not isotonic and is hemolytic
Squeeze and inspect inner bag which maintains product
sterility Discard if leaks are found Rx Only Store unit in
moisture barrier overwrap at room temperature (25ºC) until
Ready to use Avoid excessive heat See insertSterile Water
H20
For Irrigation USPUROMATIC container
Baxter Logo
Baxter Healthcare Corporation
Deerfield IL 60015 USAMade in USA
PL 146 Plastic
Baxter UROMATIC and
PL 146 are trademarks of
Baxter International IncFor product information
1-800-933-03031800
1600
1400
1200
1000
800
600
400
200 -
INGREDIENTS AND APPEARANCE
STERILE WATER
water irrigantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-0003 Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 100 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0003-44 14 in 1 CARTON 05/30/1980 1 1000 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC: 0338-0003-46 6 in 1 CARTON 05/30/1980 2 2000 mL in 1 BAG; Type 0: Not a Combination Product 3 NDC: 0338-0003-47 4 in 1 CARTON 05/30/1980 3 3000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017866 05/30/1980 STERILE WATER
water irrigantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-0004 Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 100 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0004-02 24 in 1 CARTON 12/31/1974 1 250 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC: 0338-0004-03 18 in 1 CARTON 12/31/1974 2 500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC: 0338-0004-04 12 in 1 CARTON 12/31/1974 3 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 4 NDC: 0338-0004-05 9 in 1 CARTON 12/31/1974 4 1500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017428 12/31/1974 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 059140764 ANALYSIS(0338-0003) , MANUFACTURE(0338-0003) , LABEL(0338-0003) , PACK(0338-0003) , STERILIZE(0338-0003) , API MANUFACTURE(0338-0003) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 194684502 ANALYSIS(0338-0003, 0338-0004) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 001728059 ANALYSIS(0338-0004) , MANUFACTURE(0338-0004) , LABEL(0338-0004) , PACK(0338-0004) , STERILIZE(0338-0004)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.