THERAFLU NIGHTTIME MULTI-SYMPTOM SEVERE COLD POWDER- acetaminophen, diphenhydramine hydrochloride, phenylephrine hydrochloride powder
Theraflu Nighttime Multi-Symptom Severe Cold Powder by
Drug Labeling and Warnings
Theraflu Nighttime Multi-Symptom Severe Cold Powder by is a Otc medication manufactured, distributed, or labeled by GlaxoSmithKline Consumer Healthcare Holdings (US) LLC, Patheon Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients (in each packet)
- Purposes
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
- If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting consult a doctor promptly.
Do Not Use
- in a child under 12 years of age
- if you are allergic to acetaminophen
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- with any other product containing diphenhydramine, even one used on the skin
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers
- taking the blood thinning drug warfarin
When using this product
- do not exceed recommended dosage
- avoid alcoholic drinks
- marked drowsiness may occur
- alcohol, sedatives and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occurs
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- pain, cough or nasal congestion gets worse or last more than 7 days
- cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition.
-
Directions
- do not use more than directed
- take every 4 hours, while symptoms persist. Do not take more than 6 packets in 24 hours unless directed by a doctor
- Age
- Dose
- adults and children 12 years of age and over
- one packet
- children under 12 years of age
- do not use
- dissolve contents of one packet into 8 oz. hot water; sip while hot. Consumer entire drink within 10-15 minutes.
- if using a microwave, add contents of one packet to 8 oz. of cool water; stir briskly before and after heating. Do not overheat.
- Other information
- Inactive ingredients
- Questions or Comments?
-
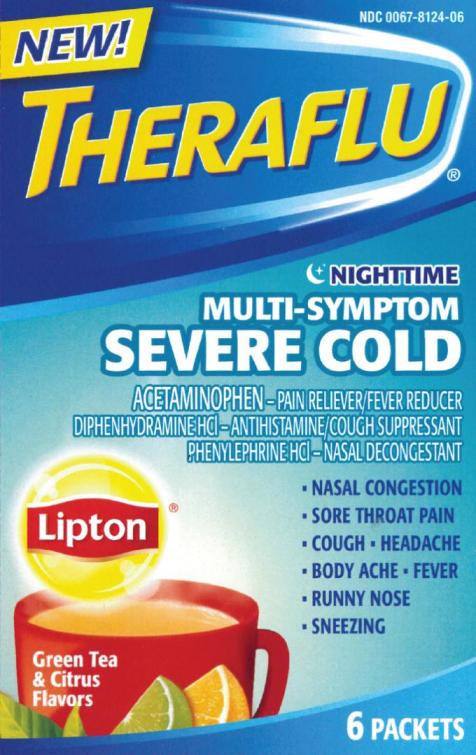
Principal Display Panel
NDC: 0067-8124-06
THERAFLU®
NIGHTTIME
MULTI-SYMPTOM
SEVERE COLD
ACETAMINOPHEN-PAIN RELIEVER/FEVER REDUCER
DIPHENHYDRAMINE HCl-ANTIHISTAMINE/COUGH SUPPRESSANT
PHENYLEPHRINE HCl-NASAL DECONGESTANT
- NASAL CONGESTION
- SORE THROAT PAIN
- COUGH HEADACHE
- BODY ACHE FEVER
- RUNNY NOSE
- SNEEZING
6 PACKETS
Theraflu® provides powerful relief from your severe cold and flu symptoms.
www.theraflu.com
READ ALL WARNINGS AND DIRECTIONS ON CARTON BEFORE USE.
KEEP CARTON FOR REFERENCE. DO NOT DISCARD.
TAMPER EVIDENT INNER UNIT
DO YOU USE IF SEALED THERAFLU PACKET IS TORN OR BROKEN.
Lipton is a registered trademark of the Unilever Group of Companies and is used under license.
Distributed by: Novartis Consumer Health, Inc.
Parsippany, NJ 07054-0622
©2015
Made in Canada
12211
-
INGREDIENTS AND APPEARANCE
THERAFLU NIGHTTIME MULTI-SYMPTOM SEVERE COLD POWDER
acetaminophen, diphenhydramine hydrochloride, phenylephrine hydrochloride powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0067-8124 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ASPARTAME (UNII: Z0H242BBR1) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MALTODEXTRIN (UNII: 7CVR7L4A2D) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CITRATE (UNII: 1Q73Q2JULR) SUCROSE (UNII: C151H8M554) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) Product Characteristics Color WHITE (white to off-white) , YELLOW (yellow, beige/brown granules) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0067-8124-06 6 in 1 CARTON; Type 0: Not a Combination Product 07/15/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 07/15/2015 Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) Establishment Name Address ID/FEI Business Operations Patheon Inc. 205475333 MANUFACTURE(0067-8124) , ANALYSIS(0067-8124) , PACK(0067-8124) , LABEL(0067-8124)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.