BETAINE ANHYDROUS FOR ORAL SOLUTION- betaine powder, for solution
Betaine Anhydrous For Oral Solution by
Drug Labeling and Warnings
Betaine Anhydrous For Oral Solution by is a Prescription medication manufactured, distributed, or labeled by Lukare Medical, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BETAINE ANHYDROUS FOR ORAL SOLUTION safely and effectively. See full prescribing information for BETAINE ANHYDROUS FOR ORAL SOLUTION.
BETAINE ANHYDROUS FOR ORAL SOLUTION
Initial U.S. Approval: 1996INDICATIONS AND USAGE
Betaine Anhydrous for Oral Solution is a methylating agent indicated in pediatric and adult patients for the treatment of homocystinuria to decrease elevated homocysteine blood concentrations. Included within the category of homocystinuria are (1):
- Cystathionine beta-synthase (CBS) deficiency
- 5,10-methylenetetrahydrofolate reductase (MTHFR) deficiency
- Cobalamin cofactor metabolism (cbl) defect
DOSAGE AND ADMINISTRATION
Adults and Pediatric Patients 3 Years of Age and Older
- The recommended dosage is 6 grams per day, administered orally in divided doses of 3 grams twice daily. (2.1)
Pediatric Patients Less than 3 Years of Age
- The recommended starting dosage is 100 mg/kg/day, administered orally in divided doses of 50 mg/kg twice daily, and then increased weekly by 50 mg/kg increments. (2.1)
- Monitor patient response by plasma homocysteine concentrations. (2.1)
- Increase the dosage gradually until the plasma total homocysteine concentration is undetectable or present only in small amounts. (2.1)
Preparation and Administration Instructions
- Prescribed amount of Betaine Anhydrous for Oral Solution should be measured with the measuring scoop provided and then dissolved in 4 to 6 ounces of water, juice, milk, or formula until completely dissolved, or mixed with food for immediate ingestion. (2.2)
DOSAGE FORMS AND STRENGTHS
For oral solution: in bottles containing 180 grams of betaine anhydrous. (3)
CONTRAINDICATIONS
- None (4)
WARNINGS AND PRECAUTIONS
- Hypermethioninemia in Patients with CBS Deficiency:
Betaine Anhydrous for Oral Solution may worsen elevated plasma methionine concentrations and cerebral edema has been reported. Monitor plasma methionine concentrations in patients with CBS deficiency. Keep plasma methionine concentrations below 1,000 micromol/L through dietary modification and, if necessary, a reduction of Betaine Anhydrous for Oral Solution dosage. (5.1)
ADVERSE REACTIONS
Most common adverse reactions (> 2%) are: nausea and gastrointestinal distress, based on physician survey. (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Lukare Medical, LLC at 1-855-752-9317, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Preparation and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypermethioninemia in Patients with CBS Deficiency
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Betaine Anhydrous for Oral Solution is indicated for the treatment of homocystinuria to decrease elevated homocysteine blood concentrations in pediatric and adult patients. Included within the category of homocystinuria are:
- Cystathionine beta-synthase (CBS) deficiency
- 5,10-methylenetetrahydrofolate reductase (MTHFR) deficiency
- Cobalamin cofactor metabolism (cbl) defect
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
Therapy with Betaine Anhydrous for Oral Solution should be directed by physicians knowledgeable in the management of patients with homocystinuria.
Adults and Pediatric Patients 3 Years of Age and Older
The recommended dosage is 6 grams per day, administered orally in divided doses of 3 grams twice daily.
Pediatric Patients Less than 3 Years of Age
The recommended starting dosage is 100 mg/kg/day divided in twice daily doses, and then increased weekly by 50 mg/kg increments.
Monitoring
Monitor patient response to Betaine Anhydrous for Oral Solution by homocysteine plasma concentration. Increase the dosage in all patients gradually until the plasma total homocysteine concentration is undetectable or present only in small amounts. An initial response in homocysteine plasma concentrations usually occurs within several days and steady state plasma concentrations occur within a month.
Monitor plasma methionine concentrations in patients with CBS deficiency [See Warnings and Precautions (5.1)].
Maximum Dosage
Dosages of up to 20 grams/day have been necessary to control homocysteine concentrations in some patients. However, one pharmacokinetic and pharmacodynamic in vitro simulation study indicated minimal benefit from exceeding a twice-daily dosing schedule and a 150 mg/kg/day dosage for Betaine Anhydrous for Oral Solution.
2.2 Preparation and Administration Instructions
- Shake bottle lightly before removing cap.
- Measure the number of scoops for the patient's dose with the scoop provided. One level scoop (1.5 mL) is equivalent to 1 gram of betaine anhydrous powder.
- Mix powder with 4 to 6 ounces (120 to 180 mL) of water, juice, milk, or formula until completely dissolved, or mix with food, then ingest mixture immediately.
- Always replace the cap tightly after using and protect the bottle from moisture.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypermethioninemia in Patients with CBS Deficiency
Patients with homocystinuria due to cystathionine beta-synthase (CBS) deficiency may also have elevated plasma methionine concentrations. Treatment with Betaine Anhydrous for Oral Solution may further increase methionine concentrations due to the remethylation of homocysteine to methionine. Cerebral edema has been reported in patients with hypermethioninemia, including patients treated with Betaine Anhydrous for Oral Solution [see Adverse Reactions (6.2)]. Monitor plasma methionine concentrations in patients with CBS deficiency. Plasma methionine concentrations should be kept below 1,000 micromol/L through dietary modification and, if necessary, a reduction of Betaine Anhydrous for Oral Solution dosage.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in labeling:
- Hypermethioninemia and cerebral edema in patients with CBS deficiency [see Warnings and Precautions (5.1)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The assessment of clinical adverse reactions is based on a survey study of 41 physicians, who treated a total of 111 homocystinuria patients with Betaine Anhydrous for Oral Solution. Adverse reactions were retrospectively recalled and were not collected systematically in this open-label, uncontrolled, physician survey. Thus, this list may not encompass all types of potential adverse reactions, reliably estimate their frequency, or establish a causal relationship to drug exposure. The following adverse reactions were reported (Table 1):
Table 1: Number of Patients with Adverse Reactions to Betaine Anhydrous for Oral Solution by Physician Survey Adverse Reactions Number of Patients Nausea
2
Gastrointestinal distress
2
Diarrhea
1
"Bad Taste"
1
"Caused Odor"
1
Questionable psychological changes
1
“Aspirated the powder”
1
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of Betaine Anhydrous for Oral Solution. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Severe cerebral edema and hypermethioninemia have been reported within 2 weeks to 6 months of starting Betaine Anhydrous for Oral Solution therapy, with complete recovery after discontinuation of Betaine Anhydrous for Oral Solution. All patients who developed cerebral edema had homocystinuria due to CBS deficiency and had severe elevation in plasma methionine concentrations (range 1,000 to 3,000 microM). As cerebral edema has also been reported in patients with hypermethioninemia, secondary hypermethioninemia due to betaine therapy has been postulated as a possible mechanism of action [see Warnings and Precautions (5.1)].
Other adverse reactions include: anorexia, agitation, depression, irritability, personality disorder, sleep disturbed, dental disorders, diarrhea, glossitis, nausea, stomach discomfort, vomiting, hair loss, hives, skin odor abnormalities, and urinary incontinence.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from a limited number of published case reports and postmarketing experience with Betaine Anhydrous for Oral Solution use in pregnancy have not identified any drug associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with betaine.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of betaine in human or animal milk, the effects on the breastfed child, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Betaine Anhydrous for Oral Solution and any potential adverse effects on the breastfed child from Betaine Anhydrous for Oral Solution or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of Betaine Anhydrous for Oral Solution have been established in pediatric patients. The majority of case studies of homocystinuria patients treated with Betaine Anhydrous for Oral Solution have been pediatric patients, including patients ranging in age from 24 days to 17 years [see Clinical Studies (14)]. Children younger than 3 years of age may benefit from dose titration [see Dosage and Administration (2.1)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
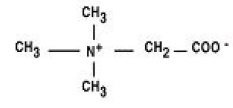
Betaine Anhydrous for Oral Solution is an agent for the treatment of homocystinuria. It contains no ingredients other than anhydrous betaine. Betaine Anhydrous for Oral Solution is a white, granular, hygroscopic powder, which is diluted in water and administered orally. The chemical name of betaine anhydrous powder is trimethylglycine. It has a molecular weight of 117.15. The structural formula is:
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Betaine Anhydrous for Oral Solution acts as a methyl group donor in the remethylation of homocysteine to methionine in patients with homocystinuria. Betaine occurs naturally in the body. It is a metabolite of choline and is present in small amounts in foods such as beets, spinach, cereals, and seafood.
12.2 Pharmacodynamics
Betaine Anhydrous for Oral Solution was observed to lower plasma homocysteine concentration in three types of homocystinuria, including CBS deficiency; MTHFR deficiency; and cbl defect. Patients have taken Betaine Anhydrous for Oral Solution for many years without evidence of tolerance. There has been no demonstrated correlation between Betaine concentration and homocysteine concentration.
In CBS-deficient patients, large increases in methionine concentration over baseline have been observed. Betaine Anhydrous for Oral Solution has also been demonstrated to increase low plasma methionine and S-adenosylmethionine (SAM) concentrations in patients with MTHFR deficiency and cbl defect.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity and fertility studies have not been conducted with Betaine Anhydrous for Oral Solution. No evidence of genotoxicity was demonstrated in the following tests: metaphase analysis of human lymphocytes; bacterial reverse mutation assay; and mouse micronucleus test.
-
14 CLINICAL STUDIES
Betaine Anhydrous for Oral Solution was studied in a double-blind, placebo-controlled, crossover study in 6 patients (3 males and 3 females) with CBS deficiency, ages 7 to 32 years at enrollment. Betaine Anhydrous for Oral Solution was administered at a dosage of 3 grams twice daily, for 12 months. Plasma homocystine concentrations were significantly reduced (p<0.01) compared to placebo. Plasma methionine concentrations were variable and not significantly different compared to placebo.
Betaine Anhydrous for Oral Solution has also been evaluated in observational studies without concurrent controls in patients with homocystinuria due to CBS deficiency, MTHFR deficiency, or cbl defect. A review of 16 case studies and the randomized controlled trial previously described was also conducted, and the data available for each study were summarized; however, no formal statistical analyses were performed. The studies included a total of 78 male and female patients with homocystinuria who were treated with Betaine Anhydrous for Oral Solution. This included 48 patients with CBS deficiency, 13 with MTHFR deficiency, and 11 with cbl defect, ranging in age from 24 days to 53 years. The majority of patients (n=48) received 6 gm/day, 3 patients received less than 6 gm/day, 12 patients received doses from 6 to 15 gm/day, and 5 patients received doses over 15 gm/day. Most patients were treated for more than 3 months (n=57) and 30 patients were treated for 1 year or longer (range 1 month to 11 years). Homocystine is formed nonenzymatically from two molecules of homocysteine, and both have been used to evaluate the effect of Betaine Anhydrous for Oral Solution in patients with homocystinuria. Plasma homocystine or homocysteine concentrations were reported numerically for 62 patients, and 61 of these patients showed decreases with Betaine Anhydrous for Oral Solution treatment. Homocystine decreased by 83 to 88% regardless of the pre-treatment concentrations, and homocysteine decreased by 71 to 83%, regardless of pre-treatment concentration. Clinical improvement, such as improvement in seizures, or behavioral and cognitive functioning, was reported by the treating physicians in about three-fourths of patients. Many of these patients were also taking other therapies such as vitamin B6 (pyridoxine), vitamin B12 (cobalamin), and folate with variable biochemical responses. In most cases, adding Betaine Anhydrous for Oral Solution resulted in a further reduction of either homocystine or homocysteine concentrations.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
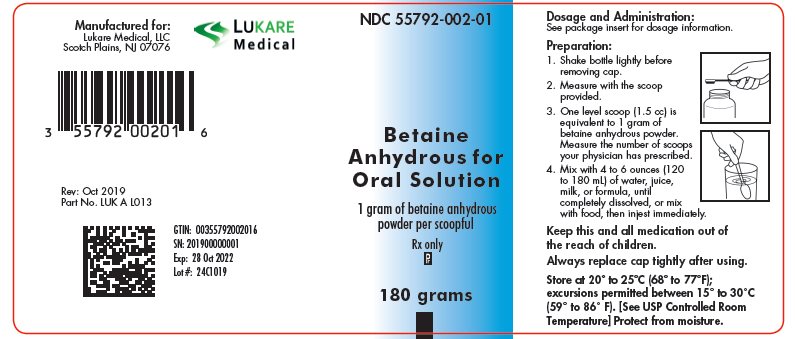
Betaine Anhydrous for Oral Solution is available in plastic bottles containing 180 grams of betaine anhydrous as a white, granular, hygroscopic powder. Each bottle is equipped with a plastic child-resistant cap and is supplied with a polypropylene measuring scoop. One level scoop (1.5 mL) is equal to 1 gram of betaine anhydrous powder.
NDC: 55792-002-01 180 g/bottle
Betaine Anhydrous for Oral Solution can be ordered by calling Lukare Medical, LLC., Customer service at 1-855-752-9317.
Storage
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Protect from moisture.
-
17 PATIENT COUNSELING INFORMATION
Preparation and Administration Instructions
Instruct patients and caregivers to administer Betaine Anhydrous for Oral Solution as follows:
- Shake bottle lightly before removing cap.
- Measure the number of scoops for the patient's dose with the scoop provided. One level scoop (1.5 mL) is equivalent to 1 gram of betaine anhydrous powder.
- Mix powder with 4 to 6 ounces (120 to 180 mL) of water, juice, milk, or formula until completely dissolved, or mix with food, then ingest mixture immediately.
- Always replace the cap tightly after using and protect bottle from moisture.
Manufactured for and Distributed by:
Lukare Medical, LLC.
Scotch Plains, NJ 07076
Part No.: LUK L 012
-
Package/Label Display Panel
NDC: 55792-002-01
Betaine Anhydrous for Oral Solution1 gram of betaine anhydrous powder per scoopful
180 grams
-
INGREDIENTS AND APPEARANCE
BETAINE ANHYDROUS FOR ORAL SOLUTION
betaine powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55792-002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BETAINE (UNII: 3SCV180C9W) (BETAINE - UNII:3SCV180C9W) BETAINE 1 g in 1 g Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55792-002-01 180 g in 1 BOTTLE; Type 0: Not a Combination Product 02/11/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210508 02/11/2022 Labeler - Lukare Medical, LLC (062862393)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.