LANEIGE NEO CUSHION MATTE 31W HONEY by Amorepacific Corporation LANEIGE NEO CUSHION
LANEIGE NEO CUSHION MATTE 31W HONEY by
Drug Labeling and Warnings
LANEIGE NEO CUSHION MATTE 31W HONEY by is a Otc medication manufactured, distributed, or labeled by Amorepacific Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
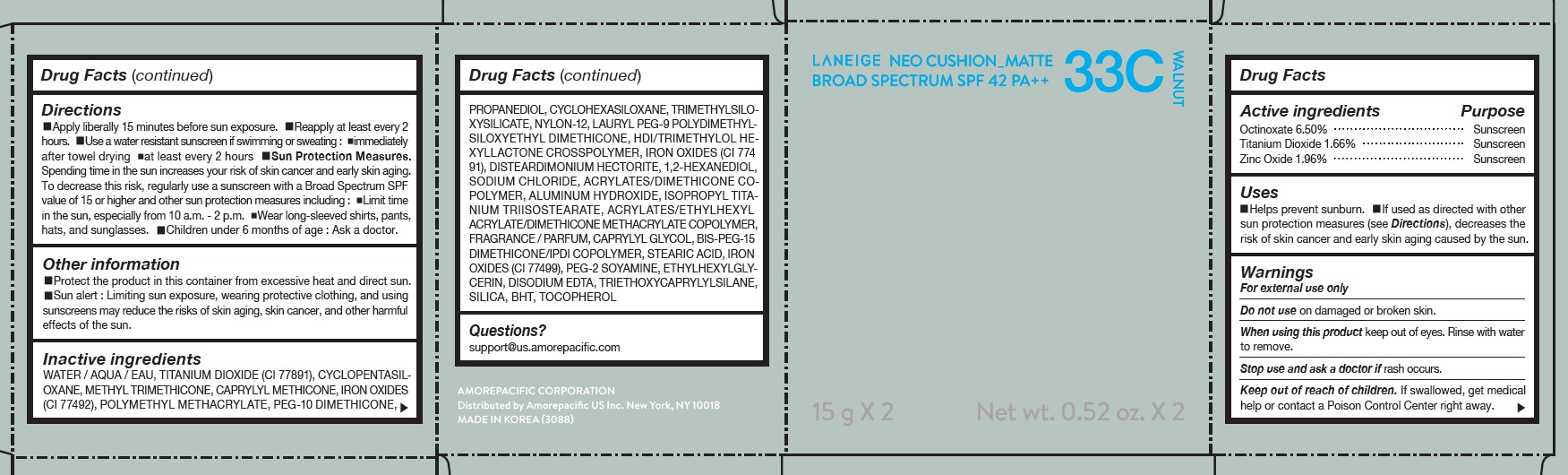
LANEIGE NEO CUSHION MATTE 33C WALNUT- zinc oxide, octinoxate, and titanium dioxide lotion
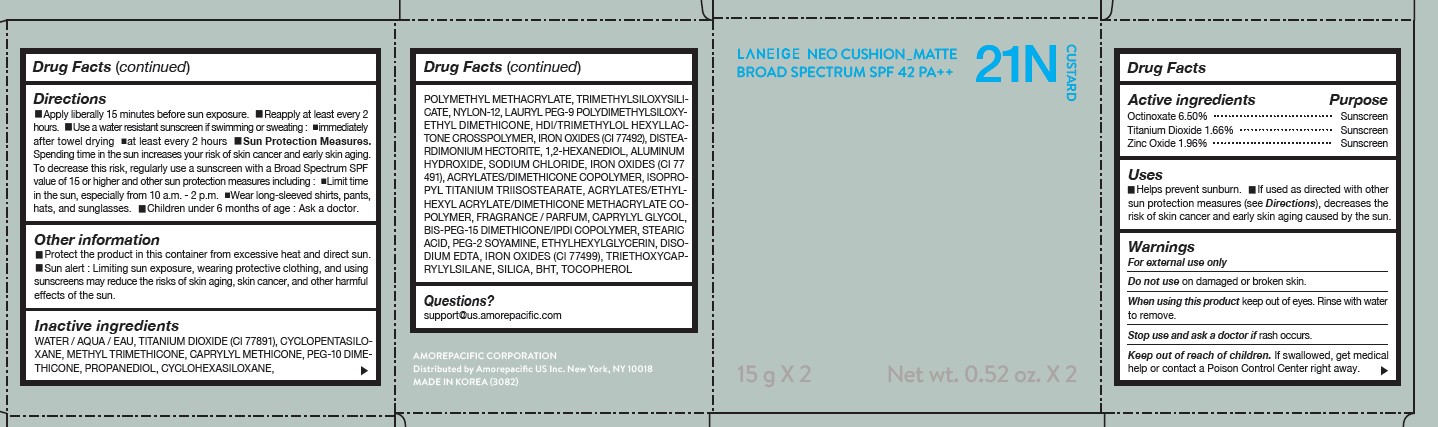
LANEIGE NEO CUSHION MATTE 21N CUSTARD- zinc oxide, octinoxate, and titanium dioxide lotion
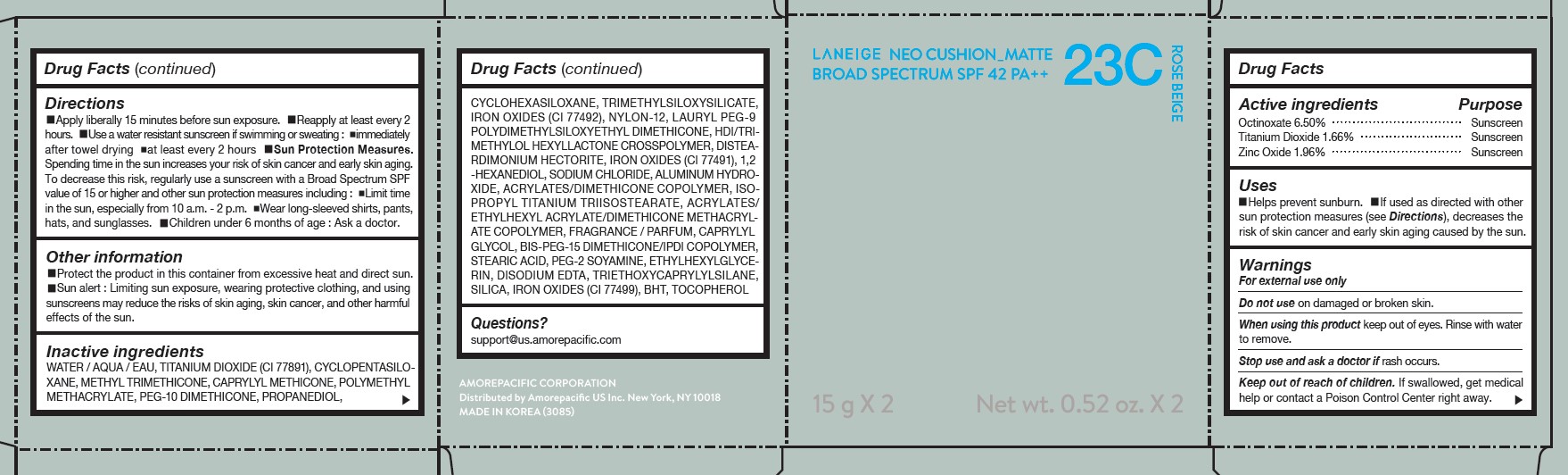
LANEIGE NEO CUSHION MATTE 23C ROSE BEIGE- zinc oxide, octinoxate, and titanium dioxide lotion
LANEIGE NEO CUSHION MATTE 33N HAZELNUT- zinc oxide, octinoxate, and titanium dioxide lotion
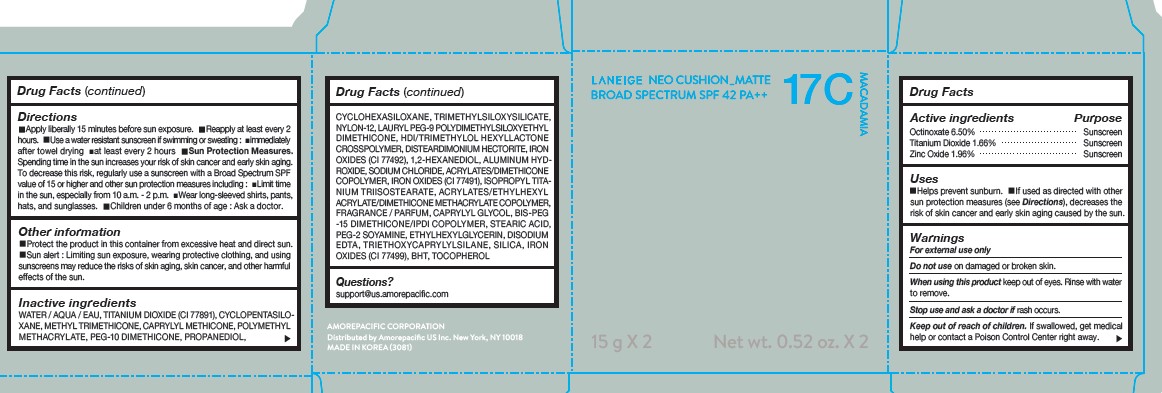
LANEIGE NEO CUSHION MATTE 17C MACADAMIA- zinc oxide, octinoxate, and titanium dioxide lotion
LANEIGE NEO CUSHION MATTE 23N CASHEW- zinc oxide, octinoxate, and titanium dioxide lotion
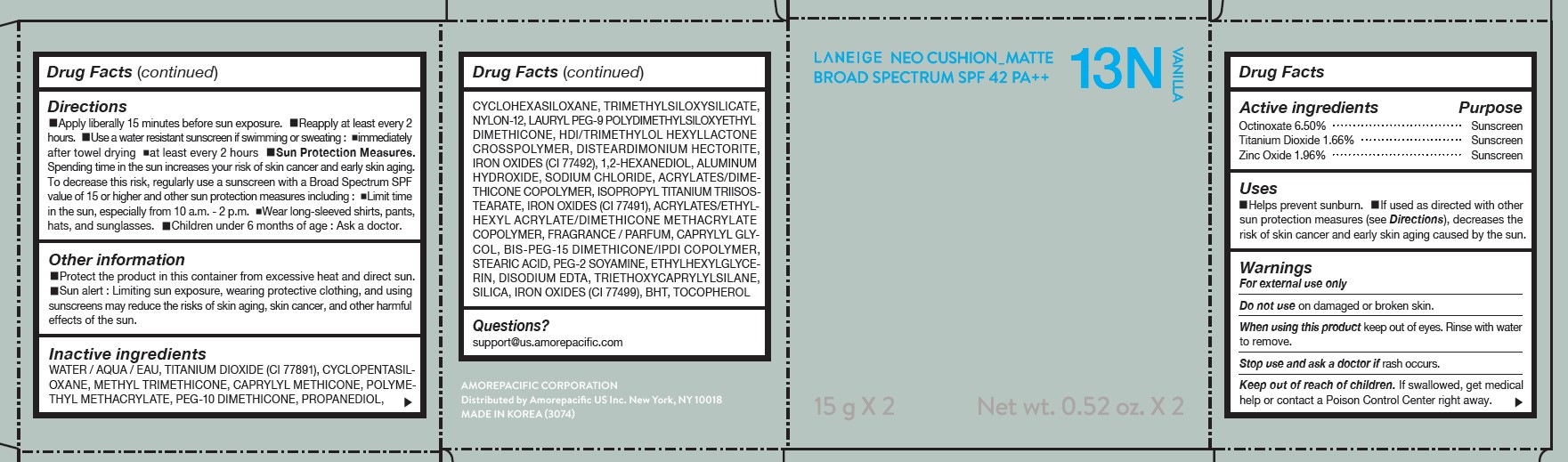
LANEIGE NEO CUSHION MATTE 13N VANILLA- zinc oxide, octinoxate, and titanium dioxide lotion
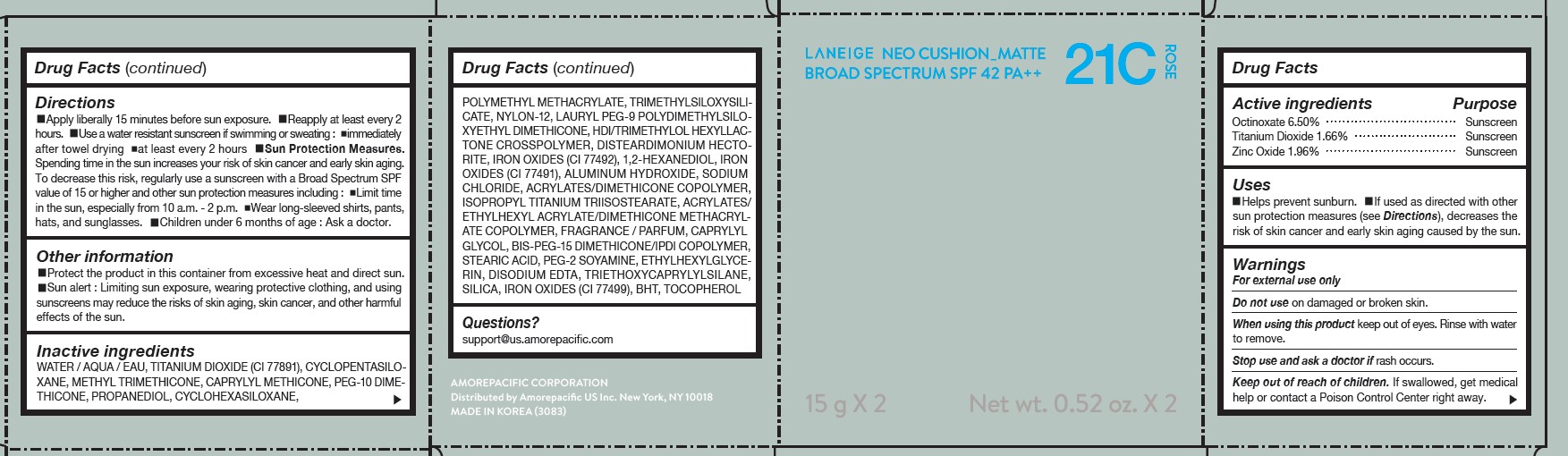
LANEIGE NEO CUSHION MATTE 21C ROSE- zinc oxide, octinoxate, and titanium dioxide lotion
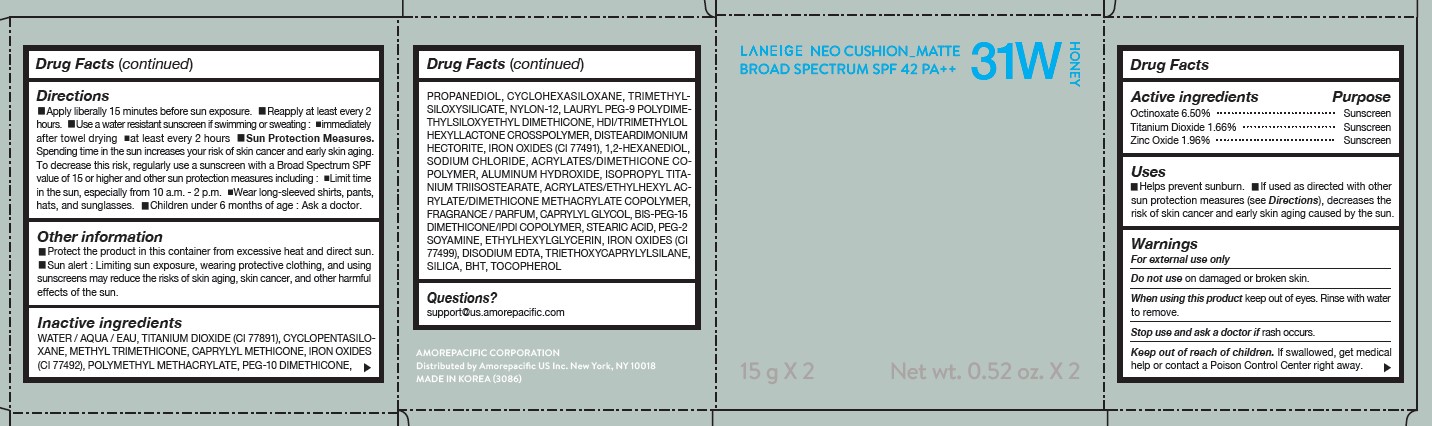
LANEIGE NEO CUSHION MATTE 31W HONEY- zinc oxide, octinoxate, and titanium dioxide lotion
Amorepacific Corporation
----------
LANEIGE NEO CUSHION
USES
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
DIRECTIONS
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging.
- To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Limit time in the sun, especially from 10 a.m.- 2 p.m. Wear long-sleeved shirts, pants, hats, and sunglasses. Children under 6 months of age: Ask a doctor.
INACTIVE INGREDIENTS
WATER / AQUA / EAU, CYCLOPENTASILOXANE, TITANIUM DIOXIDE (CI 77891), PEG-10 DIMETHICONE, CYCLOHEXASILOXANE, PHENYL TRIMETHICONE, BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE, LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE, NIACINAMIDE, ACRYLATES /ETHYLHEXYL ACRYLATE/DIMETHICONE METHACRYLATE COPOLYMER, METHYL METHACRYLATE CROSSPOLYMER, BUTYLENE GLYCOL, SODIUM CHLORIDE, ALUMINUM HYDROXIDE, IRON OXIDES (CI 77492), HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, STEARIC ACID, TRIETHOXYCAPRYLYLSILANE, PHENOXYETHANOL, FRAGRANCE / PARFUM, DISTEARDIMONIUM HECTORITE, CAPRYLYL GLYCOL, YEAST EXTRACT / FAEX / EXTRAIT DE LEVURE, ACRYLATES/STEARYL ACRYLATE/DIMETHICONE METHACRYLATE COPOLYMER, IRON OXIDES (CI 77491), DIMETHICONE, TRIMETHYLSILOXYSILICATE, POLYSORBATE 80, DISODIUM EDTA, IRON OXIDES (CI 77499), HYDROGENATED LECITHIN, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, MICA (CI 77019), SILICA, 1,2-HEXANEDIOL, POLYPROPYLSILSESQUIOXANE, CHENOPODIUM QUINOA SEED EXTRACT, MAGNESIUM SULFATE, CALCIUM CHLORIDE, CAMELLIA SINENSIS LEAF EXTRACT, MANGANESE SULFATE, ZINC SULFATE, ASCORBYL GLUCOSIDE
QUESTIONS?
- For US customers : support@us.amorepacific.com
- For Canada customers : support@ca.amorepacific.com
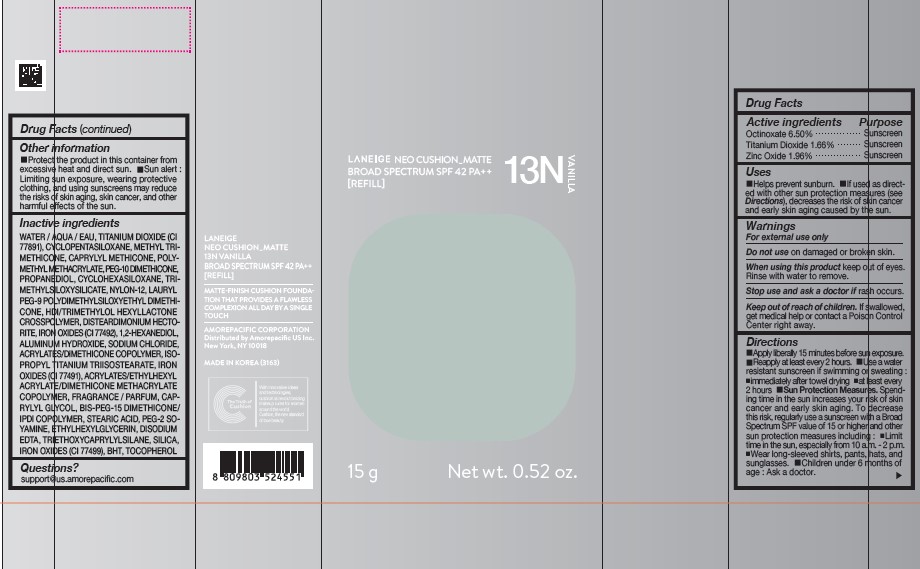
PDP - LANEIGE NEO CUSHION MATTE 13N VANILLA [Refill]
LANEIGE NEO CUSHION_MATTE 13N VANILLA
BROAD SPECTRUM SPF 42 PA ++
[REFILL]
15g Net wt. 0.52 oz.
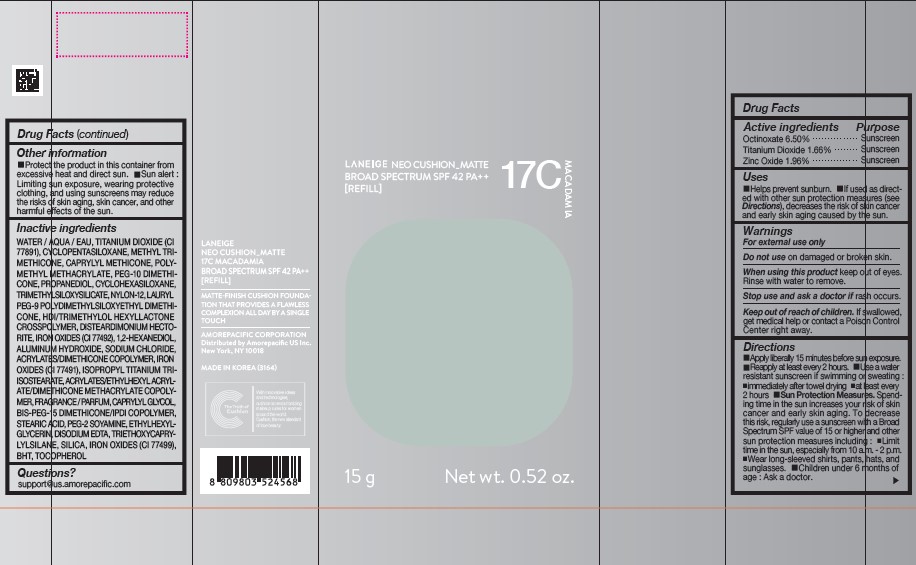
PDP - LANEIGE NEO CUSHION MATTE 17C MACADAMIA [REFILL]
LANEIGE NEO CUSHION MATTE 17C MACADAMIA
BROAD SPECTRUM SPF 42 PA ++
[REFILL]
15g Net wt. 0.52 oz.
PDP - LANEIGE NEO CUSHION MATTE 21N CUSTARD [REFILL]
LANEIGE NEO CUSHION_MATTE 21N CUSTARD
BROAD SPECTRUM SPF 42 PA ++
[REFILL]
15g Net wt. 0.52 oz.

PDP - LANEIGE NEO CUSHION MATTE 23N CASHEW [REFILL]
LANEIGE NEO CUSHION_MATTE 23N CASHEW
BROAD SPECTRUM SPF 42 PA ++
[REFILL]
15g Net wt. 0.52 oz.

PDP - LANEIGE NEO CUSHION_MATTE 23C ROSE BEIGE [REFILL]
LANEIGE NEO CUSHION_MATTE 23C ROSE BEIGE
BROAD SPECTRUM SPF 42 PA ++
[REFILL]
15g Net wt. 0.52 oz.

PDP - LANEIGE NEO CUSHION MATTE 31W HONEY [REFILL]
LANEIGE NEO CUSHION_MATTE 31W HONEY
BROAD SPECTRUM SPF 42 PA++
[REFILL]
15g Net wt. 0.52 oz.

PDP - LANEIGE NEO CUSHION_MATTE 21C ROSE [REFILL]
LANEIGE NEO CUSHION_MATTE 21C ROSE
BROAD SPECTRUM SPF 42 PA ++
[REFILL]
15g Net wt. 0.52 oz.

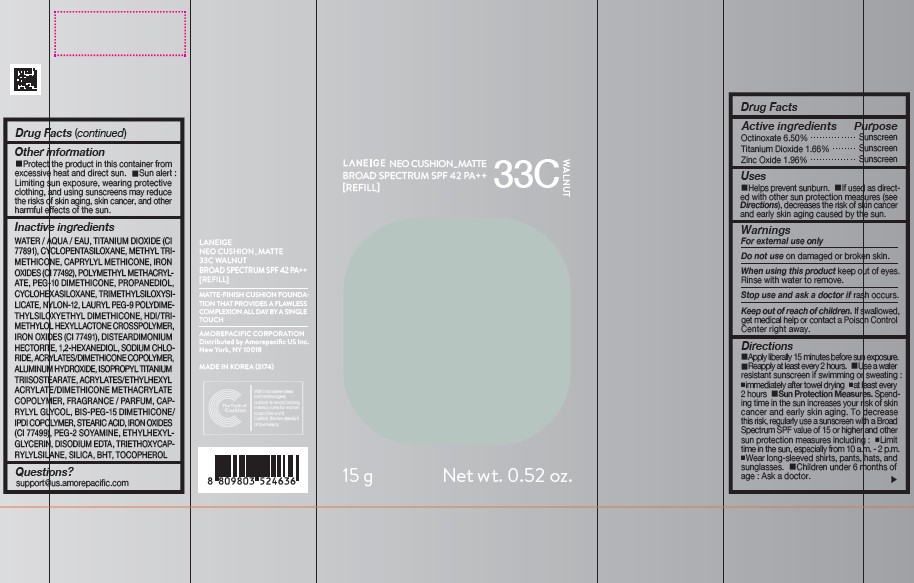
PDP - LANEIGE NEO CUSHION MATTE 33C WALNUT [REFILL]
LANEIGE NEO CUSHION_MATTE 33C WALNUT
BROAD SPECTRUM SPF 42 PA++
[REFILL]
15g Net wt. 0.52 oz.
PDP - LANEIGE NEO CUSHION MATTE 33N HAZELNUT [REFILL]
LANEIGE NEO CUSHION_MATTE 33N HAZELNUT
BROAD SPECTRUM SPF 42 PA++
[REFILL]
15g Net wt. 0.52 oz.

PDP - LANEIGE NEO CUSHION_MATTE 13N VANILLA
LANEIGE NEO CUSHION_MATTE 13N VANILLA
BROAD SPECTRUM SPF 42 PA++
15 g X 2
Net wt. 0.52 oz. X 2
PDP - LANEIGE NEO CUSHION_MATTE 17C MACADAMIA
LANEIGE NEO CUSHION_MATTE 13N VANILLA
BROAD SPECTRUM SPF 42 PA++
15 g X 2
Net wt. 0.52 oz. X 2
PDP - LANEIGE NEO CUSHION_MATTE 21N CUSTARD
LANEIGE NEO CUSHION MATTE 21N CUSTARD
BROAD SPECTRUM SPF 42 PA++
15 g X 2
Net wt. 0.52 oz. X 2
PDP - LANEIGE NEO CUSHION_MATTE 21C ROSE
LANEIGE NEO CUSHION_MATTE 21C ROSE
BROAD SPECTRUM SPF 42 PA++
15 g X 2
Net wt. 0.52 oz. X 2
PDP - LANEIGE NEO CUSHION_MATTE 23C ROSE BEIGE
LANEIGE NEO CUSHION_MATTE 23C ROSE BEIGE
BROAD SPECTRUM SPF 42 PA++
15 g X 2
Net wt. 0.52 oz. X 2
PDP - LANEIGE NEO CUSHION_MATTE 23N CASHEW
LANEIGE NEO CUSHION_MATTE 23N CASHEW
BROAD SPECTRUM SPF 42 PA++
15 g X 2
Net wt. 0.52 oz. X 2

PDP - LANEIGE NEO CUSHION_MATTE 31W HONEY
LANEIGE NEO CUSHION_MATTE 31W HONEY
BROAD SPECTRUM SPF 42 PA++
15 g X 2
Net wt. 0.52 oz. X 2
| LANEIGE NEO CUSHION MATTE 33C WALNUT
zinc oxide, octinoxate, and titanium dioxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LANEIGE NEO CUSHION MATTE 21N CUSTARD
zinc oxide, octinoxate, and titanium dioxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LANEIGE NEO CUSHION MATTE 23C ROSE BEIGE
zinc oxide, octinoxate, and titanium dioxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LANEIGE NEO CUSHION MATTE 33N HAZELNUT
zinc oxide, octinoxate, and titanium dioxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LANEIGE NEO CUSHION MATTE 17C MACADAMIA
zinc oxide, octinoxate, and titanium dioxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LANEIGE NEO CUSHION MATTE 23N CASHEW
zinc oxide, octinoxate, and titanium dioxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LANEIGE NEO CUSHION MATTE 13N VANILLA
zinc oxide, octinoxate, and titanium dioxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LANEIGE NEO CUSHION MATTE 21C ROSE
zinc oxide, octinoxate, and titanium dioxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LANEIGE NEO CUSHION MATTE 31W HONEY
zinc oxide, octinoxate, and titanium dioxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Amorepacific Corporation (631035289) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Amorepacific Corporation | 694894112 | manufacture(43419-782, 43419-783, 43419-784, 43419-785, 43419-786, 43419-787, 43419-788, 43419-789, 43419-790) | |