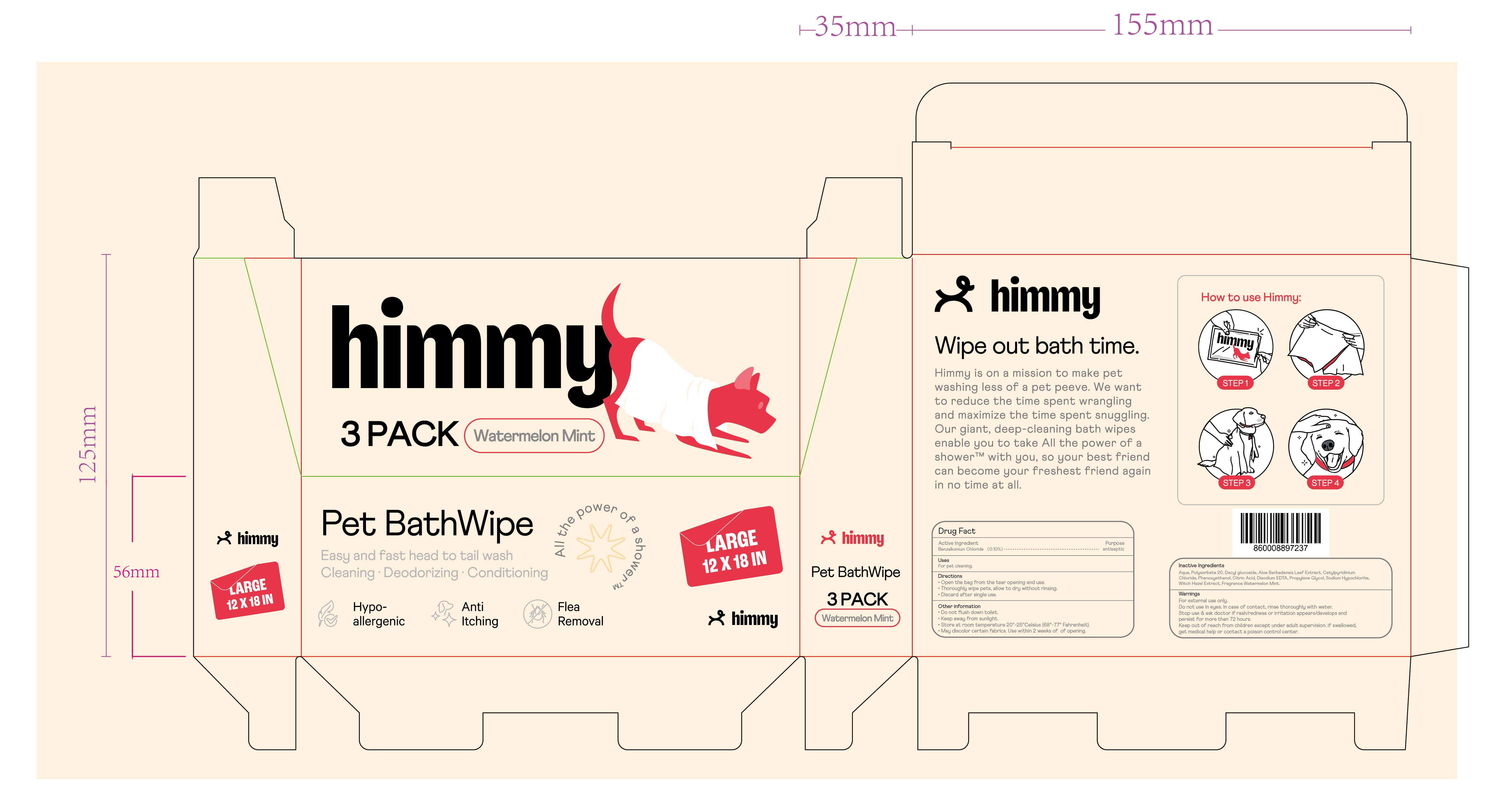
Himmy Pet BathWipe (Watermelon Mint)
Himmy Pet BathWipe by
Drug Labeling and Warnings
Himmy Pet BathWipe by is a Otc medication manufactured, distributed, or labeled by Ningbo Riway Daily Commodity Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HIMMY PET BATHWIPE- benzalkonium chloride cloth
Ningbo Riway Industrial Co., Ltd.
----------
Himmy Pet BathWipe (Watermelon Mint)
Stop use and ask doctor if rash/redness or irritation appears/develops and persist for more than 72 hours.
Keep out of reach of children except under adult supervision. If swallowed, get medical help or contact a Poison Control Center.
Directions
Open the bag from the tear opening and use.
Thoroughly wipe pets, allow to dry without rinsing.
Discard after single use.
Other information
Do not flush down toilet.
Keep away from sunlight.Store at room temperature 20°-25° Celsius(68°-77° Fahrenheit).
May discolor certain fabrics.
Use within 2 weeks of opening.
| HIMMY PET BATHWIPE
benzalkonium chloride cloth |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Ningbo Riway Industrial Co., Ltd. (540997562) |
| Registrant - Ningbo Riway Industrial Co., Ltd. (540997562) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ningbo Riway Industrial Co., Ltd. | 540997562 | manufacture(77267-012) | |