DATSCAN- ioflupane i-123 injection, solution
DaTscan by
Drug Labeling and Warnings
DaTscan by is a Prescription medication manufactured, distributed, or labeled by Medi-Physics Inc. dba GE Healthcare.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DaTscan safely and effectively. See full prescribing information for DaTscan.
DaTscan (Ioflupane I 123 Injection) for Intravenous Use
Initial U.S. Approval: 2011INDICATIONS AND USAGE
DaTscan (Ioflupane I 123 Injection) is a radiopharmaceutical indicated for striatal dopamine transporter visualization using single photon emission computed tomography (SPECT) brain imaging to assist in the evaluation of adult patients with suspected Parkinsonian syndromes (PS). In these patients, DaTscan may be used to help differentiate essential tremor from tremor due to PS (idiopathic Parkinson's disease, multiple system atrophy and progressive supranuclear palsy). DaTscan is an adjunct to other diagnostic evaluations. (1)
DOSAGE AND ADMINISTRATION
- DaTscan emits gamma radiation and must be handled with safety measures. (2.1)
- Measure patient dose by a suitable radioactivity calibration system immediately prior to administration. (2.1)
- Administer a thyroid-blocking agent at least one hour before the dose of DaTscan. (2.2)
- The recommended DaTscan dose is 111 to 185 MBq (3 to 5 mCi). (2.4)
- Begin SPECT imaging between 3 and 6 hours post-injection. (2.6)
DOSAGE FORMS AND STRENGTHS
2.5 mL of sterile solution for intravenous injection in a single-use vial [74 MBq (2 mCi)/mL at calibration time]. (3)
CONTRAINDICATIONS
Known hypersensitivity to the active substance or to any of the excipients, or to iodine. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Hypersensitivity and injection site reactions have been reported following DaTscan administration. (6.2) In clinical trials, the most common adverse reactions, headache, nausea, vertigo, dry mouth or dizziness occurred in < 1% of subjects. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GE Healthcare at 1-800-654-0118 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Amoxapine, amphetamine, benztropine, bupropion, buspirone, cocaine, mazindol, methamphetamine, methylphenidate, norephedrine, phentermine, phenylpropanolamine, selegiline, sertraline, citalopram and paroxetine may interfere with DaTscan imaging. (7) The effects of dopamine agonists and antagonists on DaTscan imaging have not been established.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety
2.2 Thyroid Blockade Before DaTscan Injection
2.3 Preparation and Administration
2.4 Recommended Dose
2.5 Radiation Dosimetry
2.6 Imaging Guidelines
2.7 Image Interpretation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Thyroid Accumulation
6 ADVERSE REACTIONS
6.1 Clinical Study Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal and Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
11.1 Physical Characteristics
11.2 External Radiation
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
DaTscan is a radiopharmaceutical indicated for striatal dopamine transporter visualization using single photon emission computed tomography (SPECT) brain imaging to assist in the evaluation of adult patients with suspected Parkinsonian syndromes (PS). In these patients, DaTscan may be used to help differentiate essential tremor from tremor due to PS (idiopathic Parkinson's disease, multiple system atrophy and progressive supranuclear palsy). DaTscan is an adjunct to other diagnostic evaluations.
-
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety
DaTscan emits radiation and must be handled with safety measures to minimize radiation exposure to clinical personnel and patients. Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experienced in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides. DaTscan dosing is based upon the radioactivity determined using a suitably calibrated instrument immediately prior to administration.
To minimize radiation dose to the bladder, encourage hydration prior to and following DaTscan administration in order to permit frequent voiding. Encourage the patient to void frequently for the first 48 hours following DaTscan administration [see Dosage and Administration (2.5)].
2.2 Thyroid Blockade Before DaTscan Injection
Before administration of DaTscan, administer Potassium Iodide Oral Solution or Lugol's Solution (equivalent to 100 mg iodide) or potassium perchlorate (400 mg) to block uptake of iodine 123 by the patient's thyroid. Administer the blocking agent at least one hour before the dose of DaTscan [see Warnings and Precautions (5.2)].
2.3 Preparation and Administration
- Assess pregnancy status before administering DaTscan to a female of reproductive potential.
- Use aseptic procedures and radiation shielding during preparation and administration. Inspect the DaTscan vial prior to administration and do not use it if the vial contains particulate matter or discoloration [see Description (11)]. Administer DaTscan as a slow intravenous injection (administered over a period of not less than 15 to 20 seconds) via an arm vein.
2.4 Recommended Dose
The recommended dose is 111 to 185 MBq (3 to 5 mCi) administered intravenously [see Clinical Studies (14)].
2.5 Radiation Dosimetry
The estimated radiation absorbed doses to an average adult from intravenous injection of DaTscan are shown in Table 1. The values are calculated assuming urinary bladder emptying at 4.8-hour intervals and appropriate thyroid blocking (iodine 123 is a known Auger electron emitter).
Table 1 Estimated Radiation Absorbed Doses from DaTscan ORGAN / TISSUE ABSORBED DOSE PER UNIT ADMINISTERED ACTIVITY (µGy / MBq) - *
The absorbed dose to the colon wall is the mass-weighted sum of the absorbed doses to the upper and lower large intestine walls, DColon = 0.57DULI + 0.43DLLI [Publication 80 of the ICRP (International Commission on Radiological Protection); Annals of the ICRP 28 (3). Oxford: Pergamon Press; 1998]
Adrenals 12.9 Brain 17.8 Striata 230 Breasts 7.8 Esophagus 10 Gallbladder Wall 26.4 GI Tract Stomach Wall 11.2 Small Intestine Wall 21.2 Colon Wall * 39.8 Upper Large Intestine Wall 38.1 Lower Large Intestine Wall 42 Heart Wall 12.9 Kidneys 10.9 Liver 27.9 Lungs 41.2 Muscle 9.4 Osteogenic Cells 28.2 Ovaries 16.8 Pancreas 13 Red Marrow 9.2 Skin 6 Spleen 10.4 Testes 8.5 Thymus 10 Thyroid 9 Urinary Bladder Wall 53.1 Uterus 16.1 Total Body 11.3 EFFECTIVE DOSE PER UNIT ADMINISTERED ACTIVITY (µSv/MBq) 21.3 The Effective Dose resulting from a DaTscan administration with an administered activity of 185 MBq (5 mCi) is 3.94 mSv in an adult.
2.6 Imaging Guidelines
Begin SPECT imaging 3 to 6 hours following DaTscan administration. Acquire images using a gamma camera fitted with high-resolution collimators and set to a photopeak of 159 keV with a ± 10% energy window. Angular sampling should be not less than 120 views over 360 degrees. Position the subject supine with the head on an off-the-table headrest, a flexible head restraint such as a strip of tape across the chin or forehead may be used to help avoid movement, and set a circular orbit for the detector heads with the radius as small as possible (typically 11 to 15 cm).
Experimental studies with a striatal phantom suggest that optimal images are obtained with matrix size and zoom factors selected to give a pixel size of 3.5 to 4.5 mm. Collect a minimum of 1.5 million counts for optimal images.
2.7 Image Interpretation
DaTscan images are interpreted visually, based upon the appearance of the striata. Reconstructed pixel size should be between 3.5 and 4.5 mm with slices 1 pixel thick. Optimum presentation of the reconstructed images for visual interpretation is transaxial slices parallel to the anterior commissure-posterior commissure (AC-PC) line. Determination of whether an image is normal or abnormal is made by assessing the extent (as indicated by shape) and intensity of the striatal signal. Image interpretation does not involve integration of the striatal image appearance with clinical signs and/or symptoms.
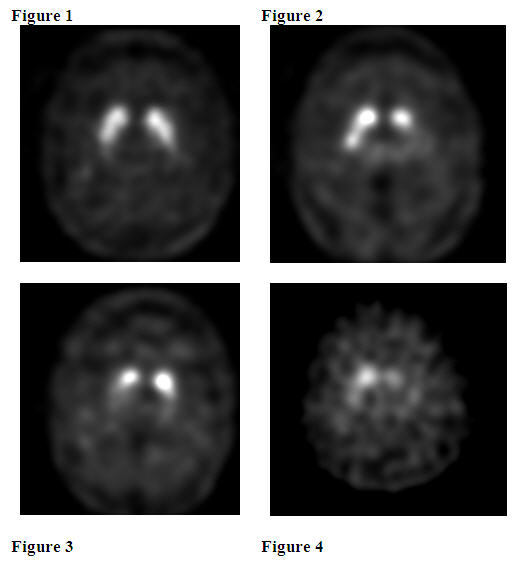
Normal:
In transaxial images, normal images are characterized by two symmetric comma- or crescent-shaped focal regions of activity mirrored about the median plane. Striatal activity is distinct, relative to surrounding brain tissue (Figure 1).
Abnormal:
Abnormal DaTscan images fall into at least one of the following three categories (all are considered abnormal).
- Activity is asymmetric, e.g. activity in the region of the putamen of one hemisphere is absent or greatly reduced with respect to the other. Activity is still visible in the caudate nuclei of both hemispheres resulting in a comma or crescent shape in one and a circular or oval focus in the other. There may be reduced activity between at least one striatum and surrounding tissues (Figure 2).
- Activity is absent in the putamen of both hemispheres and confined to the caudate nuclei. Activity is relatively symmetric and forms two roughly circular or oval foci. Activity of one or both is generally reduced (Figure 3).
- Activity is absent in the putamen of both hemispheres and greatly reduced in one or both caudate nuclei. Activity of the striata with respect to the background is reduced (Figure 4).
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions have been reported following DaTscan administration [see Adverse Reactions (6.2)]. The reactions have generally consisted of skin erythema and pruritis and have either resolved spontaneously or following the administration of corticosteroids and anti-histamines. Prior to administration, question the patient for a history of prior reactions to DaTscan. If the patient is known or strongly suspected of having had a hypersensitivity reaction to DaTscan, the decision to administer DaTscan should be based upon an assessment of the expected benefits compared to the potential hypersensitivity risks. Have anaphylactic and hypersensitivity treatment measures available prior to DaTscan administration and, following administration, observe patients for symptoms or signs of a hypersensitivity reaction.
5.2 Thyroid Accumulation
The DaTscan injection may contain up to 6% of free iodide (iodine 123). To decrease thyroid accumulation of iodine 123, block the thyroid gland before administration of DaTscan [see Dosage and Administration (2.2)]. Avoid the use of Potassium Iodide Oral Solution or Lugol's Solution in patients who are sensitive to such products. Failure to block thyroid uptake of iodine 123 may result in an increased long term risk for thyroid neoplasia.
-
6 ADVERSE REACTIONS
6.1 Clinical Study Experience
The data from clinical studies reflect exposure to DaTscan in 942 subjects with a mean age of 66 years (range 25 to 90 years). Among these subjects, 42% were women and 99% Caucasian. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of DaTscan cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. In clinical trials, no serious adverse reactions were reported. Other adverse reactions occurred at a rate of 1% or less and the reported events consisted of headache, nausea, vertigo, dry mouth or dizziness. These reactions were of mild to moderate severity.
6.2 Postmarketing Experience
Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. In the postmarketing experience, hypersensitivity reactions have been reported. The reactions generally related to rash and pruritis within minutes of DaTscan administration. The reactions either resolved spontaneously or following the administration of corticosteroids and antihistamines. Injection site pain has also been reported.
-
7 DRUG INTERACTIONS
The ioflupane within DaTscan binds to the dopamine transporter. Drugs that bind to the dopamine transporter with high affinity may interfere with the image obtained following DaTscan administration. These potentially interfering drugs consist of: amoxapine, amphetamine, benztropine, bupropion, buspirone, cocaine, mazindol, methamphetamine, methylphenidate, norephedrine, phentermine, phenylpropanolamine, selegiline, and sertraline. Selective serotonin reuptake inhibitors (paroxetine and citalopram) may increase or decrease ioflupane binding to the dopamine transporter. Whether discontinuation of these drugs prior to DaTscan administration may minimize the interference with a DaTscan image is unknown.
The impact of dopamine agonists and antagonists upon DaTscan imaging results has not been established.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Radioactive iodine products cross the placenta and can permanently impair fetal thyroid function. Administration of an appropriate thyroid blocking agent is recommended before use of DaTscan in a pregnant woman to protect the woman and fetus from accumulation of I 123 [see Dosage and Administration (2.2)].
There are no available data on DaTscan use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with ioflupane I 123. All radiopharmaceuticals have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose. Administration of DaTscan at a dose of 185 MBq (5 mCi) results in an absorbed radiation dose to the uterus of 0.3 rad (3.0 mGy). Radiation doses greater than 15 rad (150 mGy) have been associated with congenital anomalies but doses under 5 rad (50 mGy) generally have not. Advise pregnant women of the potential risks of fetal exposure to radiation doses with administration of DaTscan.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
Iodide 123 (I 123), the radionuclide in DaTscan, is present in human milk. There is no information on the effects on the breastfed infant or on milk production. Advise a lactating woman to interrupt breastfeeding and pump and discard breastmilk for at least 6 days (>10 physical half-lives) after DaTscan administration in order to minimize radiation exposure to a breastfed infant.
8.4 Pediatric Use
DaTscan is not indicated for use in children. The safety and efficacy of DaTscan have not been established in pediatric patients.
8.5 Geriatric Use
In the two principal clinical studies, 45% of the subjects were aged 65 and over. There were no differences in response compared to younger subjects that would require a dose adjustment. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
-
10 OVERDOSAGE
The clinical consequence of overdose with DaTscan has not been reported. It is unknown whether or not ioflupane is dialyzable. Due to the small quantity of ioflupane in each vial, overdosage with ioflupane is not expected to result in pharmacologic effects. The major risks of overdose relates predominantly to increased radiation exposure, with the long-term risks for neoplasia. In case of overdosage of radioactivity, frequent urination and defecation should be encouraged to minimize radiation exposure to the patient; care should be taken to avoid contamination from the radioactivity eliminated by the patient.
-
11 DESCRIPTION
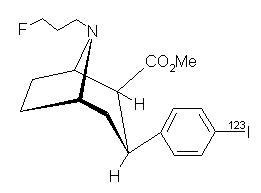
DaTscan [Ioflupane I 123 Injection] is a sterile, pyrogen-free radiopharmaceutical for intravenous injection. The clear and colorless solution is supplied in single-use vials in which each milliliter contains 0.07 to 0.13 µg ioflupane, 74 MBq (2 mCi) of iodine 123 (as ioflupane I 123) at calibration time, 5.7 mg acetic acid, 7.8 mg sodium acetate and 0.05 mL (5%) ethanol. The pH of the solution is between 4.2 and 5.2. Ioflupane I 123 has the following structural formula:
11.1 Physical Characteristics
Iodine 123 is a cyclotron-produced radionuclide that decays to 123Te by electron capture and has a physical half-life of 13.2 hours. The photon that is useful for detection and imaging studies is listed in Table 2.
Table 2 Principal Radiation Emission Data – Iodine-123 Radiation Energy Level (keV) Abundance (%) Gamma 159 83 11.2 External Radiation
The specific gamma-ray constant for iodine 123 is 1.6 R/mCi-hr at 1 cm. The first half-value thickness of lead (Pb) for iodine 123 is 0.04 cm. The relative transmission of radiation emitted by the radionuclide that results from interposition of various thicknesses of Pb is shown in Table 3 (e.g., the use of 2.16 cm Pb will decrease the external radiation exposure by a factor of about 1,000).
Table 3 Reduction in In-air Collision Kerma Caused by Lead Shielding * Shield Thickness cm of lead (Pb) Reduction in In-air Collision Kerma - * Calculation based on attenuation and energy-transfer coefficients obtained from National Institute of Standards & Technology Internal Report NISTIR 5632.
0.04 0.5 0.13 10-1 0.77 10-2 2.16 10-3 3.67 10-4 -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The active drug substance in DaTscan is N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-[123I]iodophenyl)nortropane or ioflupane I 123.
In vitro, ioflupane binds reversibly to the human recombinant dopamine transporter (DaT) (Ki = 0.62 nM; IC50 = 0.71 nM). Autoradiography of post-mortem human brain slices exposed to radiolabeled ioflupane shows concentration of the radiolabel in striatum (caudate nucleus and putamen). The specificity of the binding of ioflupane I 125 to dopamine transporter was demonstrated by competition studies with the DaT inhibitor GBR 12909 (a dopamine reuptake inhibitor), the serotonin reuptake inhibitor citalopram, and the norepinephrine reuptake inhibitor desipramine in post-mortem human brain slices exposed to radiolabeled ioflupane. Citalopram reduced binding in the neocortex and thalamus with only minor effects in the striatum. This indicated that the binding in the cortex and thalamus is mainly to the serotonin reuptake sites. Desipramine showed no effect on the level of striatal binding of ioflupane I 125, but reduced extrastriatal binding by 60 to 85%. The binding of ioflupane I 125 to the striatum was abolished in the presence of high concentrations of GBR 12909, indicating selectivity of ioflupane binding for the pre-synaptic DaT.
Following administration of DaTscan to humans, radioactive decay of the iodine 123 emits gamma radiation which can be detected externally using gamma detectors, allowing visualization of the brain striata through SPECT imaging [see Clinical Pharmacology (12.3)].
12.2 Pharmacodynamics
As DaTscan contains a very small quantity of ioflupane, no ioflupane pharmacologic effects are expected [see Description (11)].
12.3 Pharmacokinetics
The pharmacokinetics of ioflupane I 123 were studied by monitoring radioactivity following intravenous injection; only 5% of the administered radioactivity remained in whole blood at 5 minutes post-injection. Uptake in the brain reached approximately 7% of injected radioactivity at 10 minutes post-injection and decreased to 3% after 5 hours; striata to background ratios were relatively constant between 3 and 6 hours post-injection. About 30% of the whole brain radioactivity was attributed to striatal uptake. By 48 hours post-injection, approximately 60% of the injected radioactivity has been excreted in the urine, with fecal excretion estimated to be approximately 14%.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies on reproductive toxicity have not been conducted. Ioflupane showed no evidence of mutagenic potential in in vitro or in vivo mutagenicity studies. Studies to assess the carcinogenic potential of ioflupane have not been performed.
13.2 Animal Toxicology and/or Pharmacology
Single- and repeated-dose intravenous toxicity studies have been performed using ioflupane in rats, rabbits, and dogs. Additionally, single-dose acute toxicity studies have been performed in cynomolgus monkeys. No mortality or other toxicity was observed at doses up to 5,500 times the maximum clinical dose of DaTscan; at doses greater than 1,500 times the maximum clinical dose, pharmacological responses such as mydriasis and hyperactivity were seen in some species.
-
14 CLINICAL STUDIES
The safety and efficacy of DaTscan were evaluated in two multicenter, single-arm studies (Study 1 and Study 2) that evaluated 284 adult patients with tremor. In the studies, DaTscan image outcomes were compared to a reference clinical diagnostic standard of "PS" or "non-PS". The reference clinical diagnostic standard for "PS" consisted of the following diagnoses: Parkinson's disease (PD), multiple system atrophy (MSA), and progressive supranuclear palsy (PSP). These three conditions have been associated with dopaminergic neurodegeneration and DaTscan imaging was not designed to distinguish among the conditions. The reference clinical diagnostic standard for "non-PS" consisted of an essential tremor (ET) diagnosis or other non-PS diagnosis. Three to 6 hours after DaTscan administration, subjects underwent SPECT imaging with a variety of multi-headed cameras or a multi-detector single-slice systems. The median administered activity evaluated in clinical studies was 173 MBq (4.7 mCi) [range, 88 to 287 MBq (2.4 to 7.8 mCi)].
DaTscan images were evaluated by readers blinded to clinical information. Study 1 readers had no other role in patient assessment; Study 2 readers included site investigators. The reference clinical diagnostic standards were the clinical diagnoses established by a consensus panel of movement disorder specialists that evaluated data inclusive through 36 months of follow-up (Study 1) or the investigator-determined baseline clinical diagnosis (Study 2). Study 1 consisted of patients with early features of Parkinsonism; patients with features suggestive of MSA or PSP were excluded. Study 2 consisted of patients with clinically established diagnosis of PS (PD, MSA, PSP) or ET.
Among the 99 patients in Study 1, 44% were female, 42% were aged 65 or over and all were Caucasian; among the 185 patients in Study 2, 35% were female, 48% were aged 65 or over and 99% were Caucasian. Among the patients in Study 1, the baseline clinical diagnoses consisted of: probable PD (44%), possible PD (31%), "benign" PD (6%), possible ET (11%), and other diagnoses (7%). Among the patients in Study 2, the baseline clinical diagnoses consisted of: PD (70%), ET (15%), MSA (10%), and PSP (5%).
Table 4 shows the positive percent agreement and negative percent agreement of the DaTscan image results with the reference clinical diagnostic standard. Positive percent agreement represents the percent of patients with abnormal DaTscan images among all the patients with a clinical diagnostic reference standard of PS. The negative percent agreement represents the percent of patients with normal DaTscan images among the patients with a non-PS clinical diagnostic reference standard.
Table 4: Positive and Negative Percent Agreements for Studies 1 and 2 Positive percent agreement (95 % CI)
(% patients with an abnormal DaTscan image among patients with PS)Negative percent agreement (95 % CI)
(% patients with a normal DaTscan image among patients with non-PS)Study 1 (patients with early signs and/or symptoms of PS) Reader A, n = 99 77 (66, 87) 96 (82, 100) Reader B, n = 96 78 (66, 87) 96 (82, 100) Reader C, n = 98 79 (67, 87) 96 (82, 100) Study 2 (patients with established diagnoses of PS or ET) Reader A, n = 185 93 (88, 97) 96 (81, 100) Reader B, n = 185 97 (93, 99) 74 (54, 89) Reader C, n = 185 96 (92, 99) 85 (66, 96) Reader D, n = 185 92 (87, 96) 93 (76, 99) Reader E, n = 185 94 (90, 97) 93 (76, 99) The effectiveness of DaTscan as a screening or confirmatory test and for monitoring disease progression or response to therapy has not been established.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
DaTscan is supplied in 10-mL glass vials containing a total volume of 2.5 mL of solution with a total radioactivity of 185 MBq (5 mCi) at calibration time. Each vial is enclosed in a lead container of appropriate thickness.
NDC: 17156-210-01
-
17 PATIENT COUNSELING INFORMATION
Instruct patients to inform their physician or healthcare provider if they:
- are pregnant. Advise a pregnant woman of the potential risks of fetal exposure to radiation doses with DaTscan [see Use in Specific Populations (8.1)].
- are breast feeding. Advise a lactating woman to interrupt breastfeeding and pump and discard breastmilk for at least 6 days (>10 physical half-lives) after DaTscan administration in order to minimize radiation exposure to a breastfed infant [see Use in Specific Populations (8.2)].
- are sensitive to DaTscan.
- are sensitive to Potassium Iodide Oral Solution, or Lugol's Solution.
- have reduced renal or hepatic function.
Instruct patients to increase their level of hydration prior to and after receiving DaTscan and to void frequently for the first 48 hours following DaTscan administration.
-
SPL UNCLASSIFIED SECTION
Manufactured and Distributed by GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A.
DaTscan is a trademark of GE Healthcare or one of its subsidiaries.
GE and the GE Monogram are trademarks of General Electric Company.
© 2020 General Electric Company – All rights reserved.
43-2010D
Revised March 2020
-
PRINCIPAL DISPLAY PANEL - 2.5 mL Vial Label
GE Healthcare
NDC: 17156-210-01DaTscan™
Ioflupane I 123 InjectionIoflupane I 123 185 MBq (5 mCi) in 2.5 mL
at calibration.Radiopharmaceutical for Intravenous Injection.
Single Use Vial
Rx ONLY
Store at 20°-25°C (68°-77°F).
Expires 7 hours after calibration
time.Each mL contains 74 MBq (2 mCi)
of Ioflupane I 123 at calibration,
0.07 - 0.13 µg ioflupane, 5.7 mg
acetic acid, 7.8 mg sodium acetate,
0.05 mL ethanol.See package insert for dosage
and administration.CAUTION
RADIOACTIVE
MATERIALGE Healthcare
Medi-Physics, Inc.
Arl. Hgts, IL 60004
800-654-0118Product No. 2010
Est. Lic. No. 100129-A41-2010A
VOLUME:
2.5 mLRADIOACTIVE
CONCENTRATION:
74 MB q/mL
(2 mC i/mL)TOTAL
ACTIVITY:
185 MBq
(5 mCi)CALIB.
DATE:CALIB.
TIME:
1200LOT
NO.:
2010 –
-
INGREDIENTS AND APPEARANCE
DATSCAN
ioflupane i-123 injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17156-210 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ioflupane I-123 (UNII: 3MM99T8R5Q) (ioflupane I-123 - UNII:3MM99T8R5Q) ioflupane I-123 2 mCi in 1 mL Inactive Ingredients Ingredient Name Strength acetic acid (UNII: Q40Q9N063P) 5.7 mg in 1 mL sodium acetate (UNII: 4550K0SC9B) 7.8 mg in 1 mL alcohol (UNII: 3K9958V90M) 0.05 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17156-210-01 1 in 1 CONTAINER 03/01/2011 1 2.5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022454 03/01/2011 Labeler - Medi-Physics Inc. dba GE Healthcare. (095263729) Establishment Name Address ID/FEI Business Operations Medi-Physics Inc. dba GE Healthcare. 095263729 MANUFACTURE(17156-210)
Trademark Results [DaTscan]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DATSCAN 85029347 4154635 Live/Registered |
GE Healthcare Limited 2010-05-04 |
 DATSCAN 77731175 3689426 Live/Registered |
Industrial Process Systems, Inc. 2009-05-07 |
 DATSCAN 75682377 2778837 Dead/Cancelled |
GE HEALTHCARE LIMITED 1999-04-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.