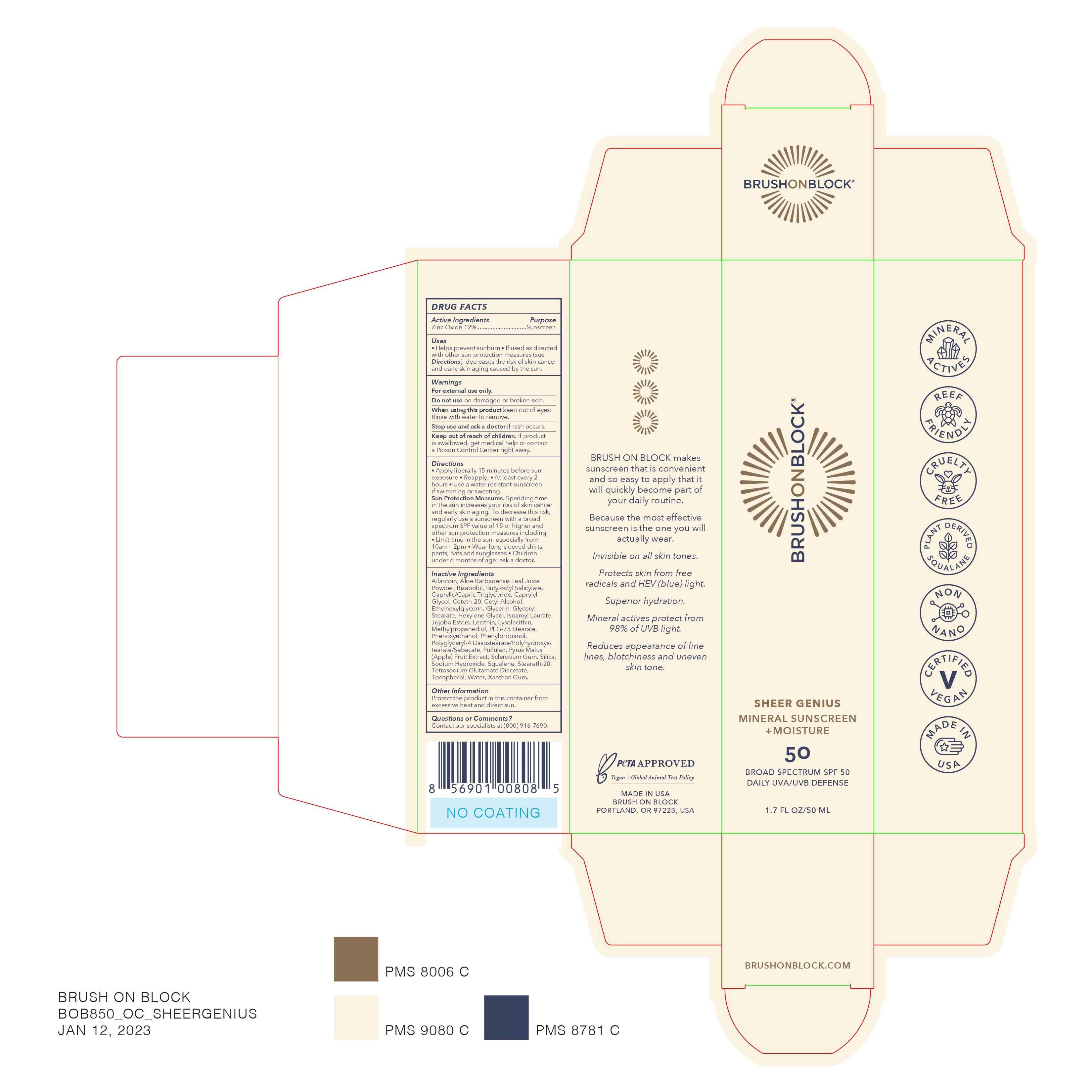
Brush on Block Sheer Genius
Brush on Block Sheer Genius Broad Spectrum SPF 50 by
Drug Labeling and Warnings
Brush on Block Sheer Genius Broad Spectrum SPF 50 by is a Otc medication manufactured, distributed, or labeled by SPF Ventures, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BRUSH ON BLOCK SHEER GENIUS BROAD SPECTRUM SPF 50- zinc oxide 12% lotion
SPF Ventures, LLC
----------
Brush on Block Sheer Genius
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early aging caused by the sun
Directions
- apply liberally 15 minutes before sun exposure
- Reapply: at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating.
- Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including: Limit time in the sun, especially from 10am - 2pm, wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor
Directions
apply liberally 15 minutes before sun exposure
Reapply: at least every 2 hours
Use a water resistant sunscreen if swimming or sweating.
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including: Limit time in the sun, especially from 10am - 2pm, wear long-sleeved shirts, pants, hats, and sunglasses
Children under 6 months: Ask a doctor
Inactive Ingredients
Allantoin, Aloe Barbadensis Leaf Juice Powder, Bisabolol, Butyloctyl Salicylate, Caprylic/Capric Tryglyceride, Caprylyl Glycol, Ceteth-20, Cetyl Alcohol, Ethylhexylglycerin, Glycerin, Glyceryl Stearate, Hexyylene Glycol, Isoamyl Laurate, Jojoba Esters, Lecithin, Lysolecithin, Methylpropanediol, PEG-75 Stearate, Phenoxyethanol, Phenylpropanol, Polyglyceryl-4 Diisostearate/Polyhydroxystearate/Sebacate, Pullulan, Pyrus Malus (Apple) Fruite Extract, Sclerotium Gum. Silica, Sodium Hydroxide, Squalene, Steareth-20, Tetrasodium Glutamate Diacetate, Tocopherol, Water, Xanthan Gum.
| BRUSH ON BLOCK SHEER GENIUS BROAD SPECTRUM SPF 50
zinc oxide 12% lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - SPF Ventures, LLC (055483891) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.