POSIMIR (Bupivacaine Solution) 660 mg/5mL (132 mg/mL)
POSIMIR by
Drug Labeling and Warnings
POSIMIR by is a Prescription medication manufactured, distributed, or labeled by Renaissance Lakewood, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
POSIMIR- bupivacaine injection
Renaissance Lakewood, LLC
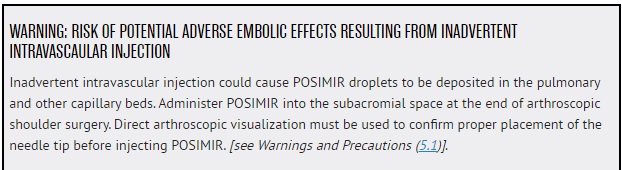
----------
POSIMIR (Bupivacaine Solution) 660 mg/5mL (132 mg/mL)
| POSIMIR
bupivacaine injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Renaissance Lakewood, LLC (077744035) |
| Registrant - Renaissance Lakewood, LLC (077744035) |
Revised: 10/2024
Document Id: 2474c73d-640a-16a7-e063-6394a90af357
Set id: efb90d91-f984-294d-e053-2a95a90ad3c4
Version: 2
Effective Time: 20241014
Trademark Results [POSIMIR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 POSIMIR 85785555 4918452 Live/Registered |
DURECT CORPORATION 2012-11-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 Product Carton
Product Carton
