QSR Foaming Alcohol Hand Sanitizer by Kay Chemical Company Drug Facts
QSR Foaming Alcohol Hand Sanitizer by
Drug Labeling and Warnings
QSR Foaming Alcohol Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Kay Chemical Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
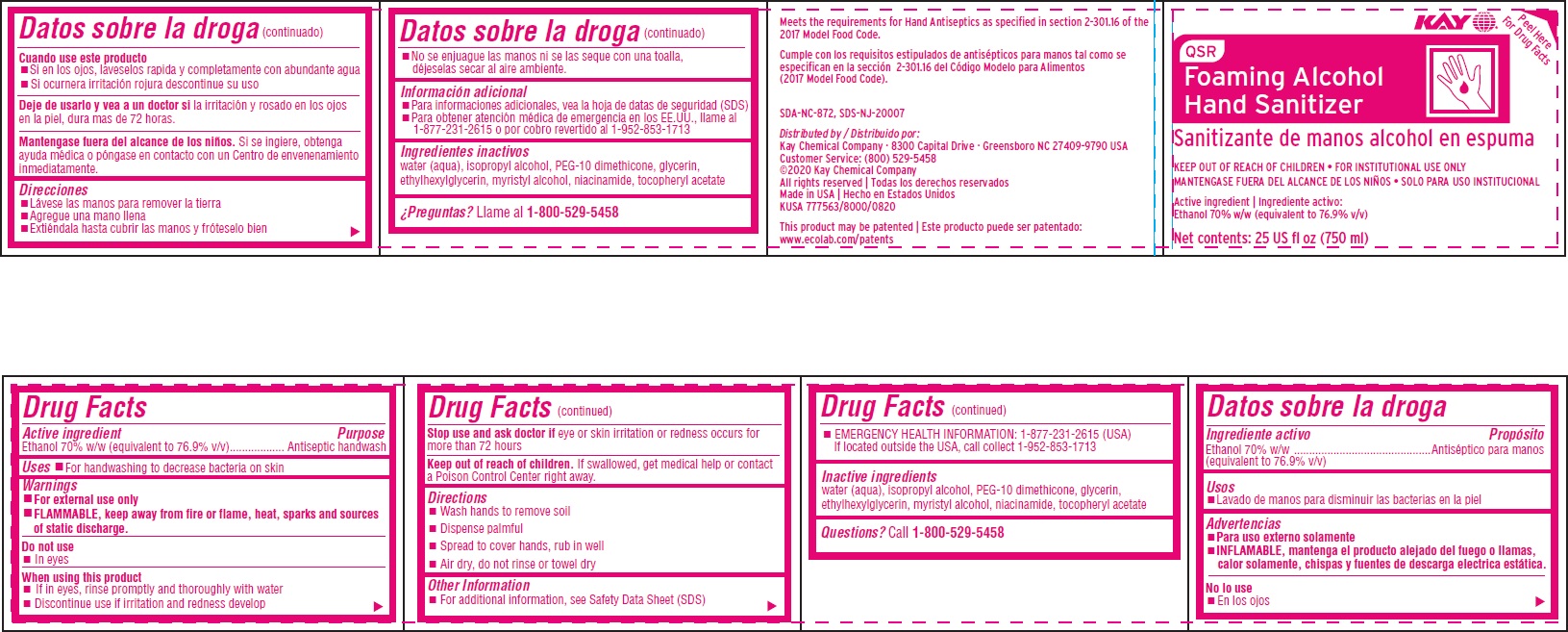
QSR FOAMING ALCOHOL HAND SANITIZER- alcohol solution
Kay Chemical Company
----------
Drug Facts
Warnings
- For external use only
- FLAMMABLE, keep away from fire or flame, heat, sparks and sources of static discharge
Directions
- wash hands to remove soil
- dispense palmful
- spread to cover hands, rub in well
- air dry, do not rinse or towel dry
Other information
- for additional information, see Safety Data Sheet (SDS)
- EMERGENCY HEALTH INFORMATION: 1-877-231-2615 (USA) If located outside the USA, call collect 1-952-853-1713
Inactive ingredients water (aqua), isopropyl alcohol, PEG-10 dimethicone, glycerin, ethylhexylglycerin, myristyl alcohol, niacinamide, tocopheryl acetate
Principal display panel and representative label
QSR
Foaming Alcohol
Hand Sanitizer
Sanitizante de manos alcohol en espuma
KEEP OUT OF REACH OF CHILDREN FOR INSTITUTIONAL USE ONLY
MANTENGASE FUERA DEL ALCANCE DE LOS NIÑOS SOLO PARA USO INSTITUCIONAL
Active ingredient | Ingrediente activo:
Ethanol 70% w/w (equivalent to 76.9% v/v)
Net contents: 25 US fl oz (750 ml)
Meets the requirements for Hand Antiseptics as specified in section 2-301.16 of the 2017 Model Food Code.
Cumple con los requisitos estipulados de antisépticos para manos tal como se especifican en la sección 2-301.16 del Código Modelo para limentos (2017 Model Food Code).
SDA-NC-872, SDS-NJ-20007
Distributed by / Distribuido por:
Kay Chemical Company · 8300 Capital Drive · Greensboro NC 27409-9790 USA Customer Service: (800) 529-5458
©2020 Kay Chemical Company
All rights reserved | Todas los derechos reservados
Made in USA | Hecho en Estados Unidos
KUSA 777563/8000/0820
This product may be patented | Este producto puede ser patentado:
| QSR FOAMING ALCOHOL HAND SANITIZER
alcohol solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Kay Chemical Company (003237021) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.