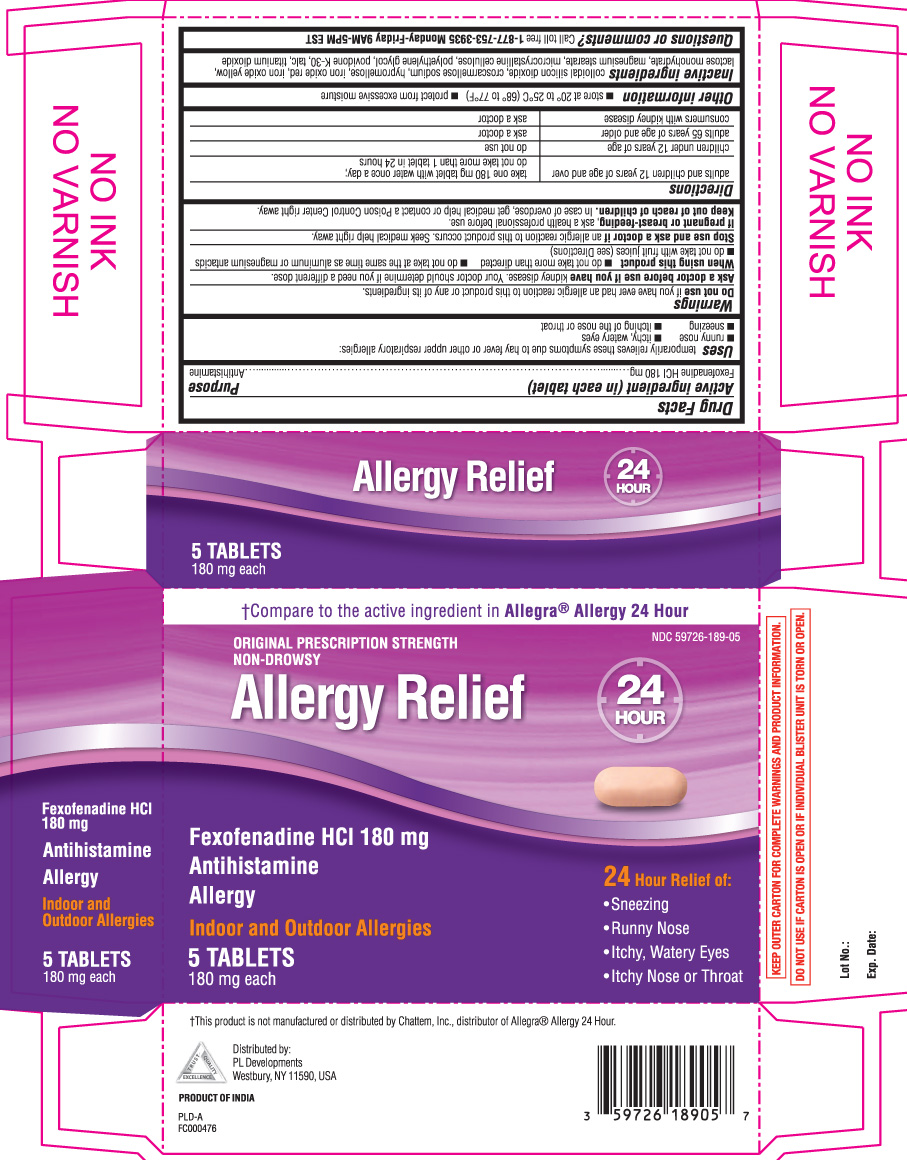
ALLERGY RELIEF- fexofenadine hcl tablet
Allergy Relief by
Drug Labeling and Warnings
Allergy Relief by is a Otc medication manufactured, distributed, or labeled by P and L Development of New York Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Package/Label Principal Display Panel
†Compare to the active ingredient in Allegra® Allergy 24 hour
ORIGINAL PRESCRIPTION STRENGTH
NON-DROWSY
ALLERGY RELIEF
Fexofenadine HCl 180 mg
Antihistamine
ALLERGY
Indoor and outdoor Allergies
24 Hour Relief of:
- sneezing
- runny nose
- itchy, watery eyes
- itchy nose or throat
†This product is not manufactured or distributed by Chattem Inc., distributor of Allegra® Allergy 24 hour
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
DO NOT USE IF CARTON IS OPEN OR IF INDIVIDUAL BLISTER UNIT IS TORN OR OPEN.
Distributed by: PL Developments
200 hicks street
westbury NY 11590
PRODUCT OF INDIA
- Product Label
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
fexofenadine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59726-189(NDC:55648-987) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POVIDONE K30 (UNII: U725QWY32X) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (light peach) Score no score Shape CAPSULE Size 17mm Flavor Imprint Code W987 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59726-189-05 1 in 1 CARTON 1 5 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079112 03/28/2013 Labeler - P and L Development of New York Corporation (800014821) Registrant - P and L Development of New York Corporation (800014821)
Trademark Results [Allergy Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLERGY RELIEF 98236984 not registered Live/Pending |
Dmytro Kononenko 2023-10-24 |
 ALLERGY RELIEF 90457167 not registered Live/Pending |
American Textile Company, Inc. 2021-01-10 |
 ALLERGY RELIEF 78838437 3358249 Live/Registered |
Meshbesher Health Corporation 2006-03-16 |
 ALLERGY RELIEF 76619855 3066888 Live/Registered |
AMERICAN TEXTILE COMPANY 2004-11-09 |
 ALLERGY RELIEF 74668018 not registered Dead/Abandoned |
NaturaLife Corporation 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.