Fair Light Tinted Drops by Make2Give LLC Fair/Light Tinted Drops
Fair Light Tinted Drops by
Drug Labeling and Warnings
Fair Light Tinted Drops by is a Otc medication manufactured, distributed, or labeled by Make2Give LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FAIR LIGHT TINTED DROPS- titanium dioxide, zinc oxide lotion
Make2Give LLC
----------
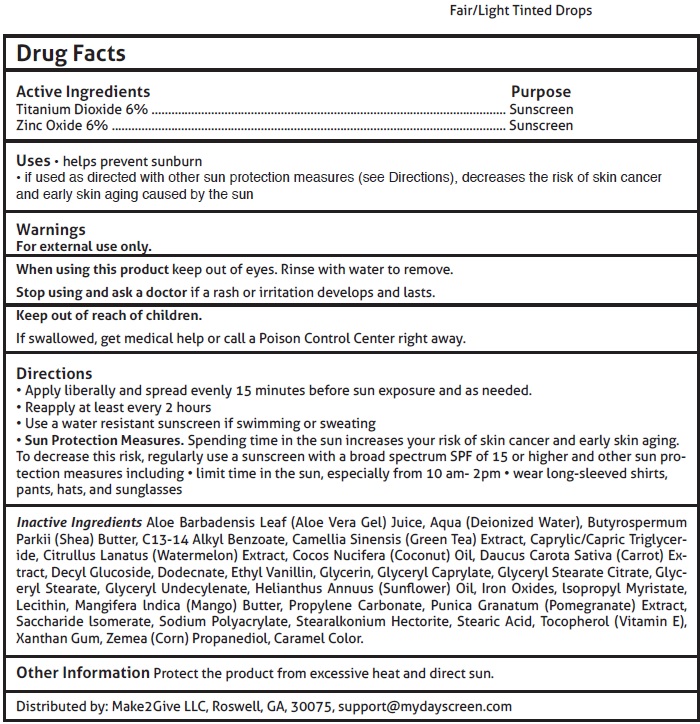
Fair/Light Tinted Drops
Uses
helps prevent sunburn
if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
Apply liberally and spread evenly 15 minutes before sun exposure and as needed.
Reapply at least every 2 hours
Use a water resistant sunscreen if swimming or sweating
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging.
To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including limit time in the sun, especially from 10 am- 2pm wear long-sleeved shirts, pants, hats, and sunglasses
Inactive Ingredients
Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Butyrospermum Parkii (Shea) Butter, C13-14 Alkyl Benzoate, Camellia Sinensis (Green Tea) Extract, Caprylic/Capric Triglyceride, Citrullus Lanatus (Watermelon) Extract, Cocos Nucifera (Coconut) Oil, Daucus Carota Sativa (Carrot) Extract, Decyl Glucoside, Dodecnate, Ethyl Vanillin, Glycerin, Glyceryl Caprylate, Glyceryl Stearate Citrate, Glyceryl Stearate, Glyceryl Undecylenate, Helianthus Annuus (Sunflower) Oil, Iron Oxides, lsopropyl Myristate, Lecithin, Mangifera lndica (Mango) Butter, Propylene Carbonate, Punica Granatum (Pomegranate) Extract, Saccharide lsomerate, Sodium Polyacrylate, Stearalkonium Hectorite, Stearic Acid, Tocopherol (Vitamin E), Xanthan Gum, Zemea (Corn) Propanediol, Caramel Color.
| FAIR LIGHT TINTED DROPS
titanium dioxide, zinc oxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Make2Give LLC (023910159) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.