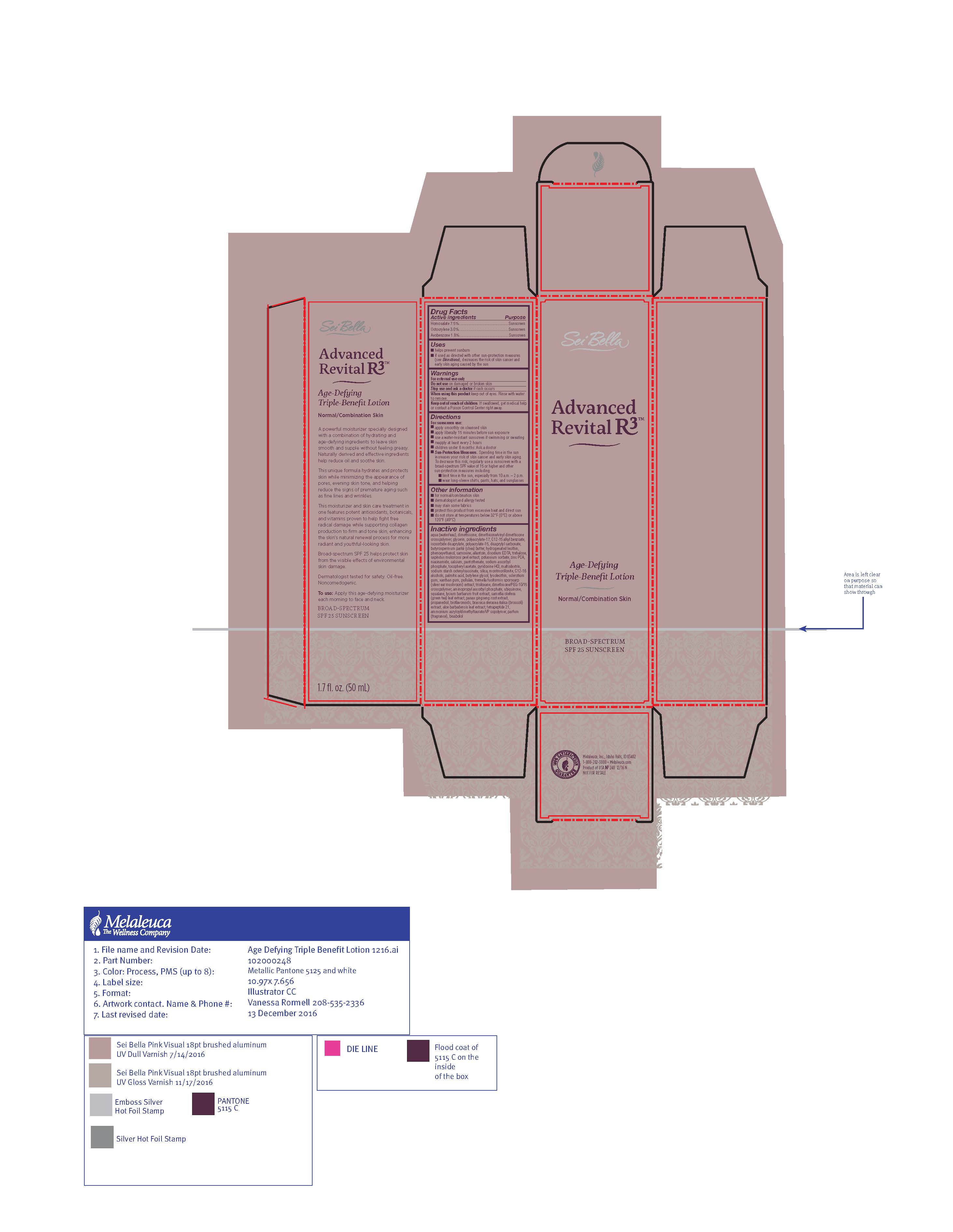
ADVANCED REVITAL- avobenzone, homosalate, octocrylene lotion
Advanced Revital by
Drug Labeling and Warnings
Advanced Revital by is a Otc medication manufactured, distributed, or labeled by Melaleuca, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- CLINICAL STUDIES
- GENERAL PRECAUTIONS
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients
aqua (water/eau), polyacrylate-15, polyacrylate-17, C20-22 alkyl phosphate, C20-22 alcohols, trisiloxane, dimethicone, cyclopentasiloxane, dimethicone crosspolymer, cetyl palmitate, glycerin, butyrospermum parkii (shea butter), C12-15 alkyl benzoate, dicaprylyl carbonate, C14-22 alcohols, C12-20 alkyl glucoside, propylheptyl caprylate, acetyl tetrapeptide-9, phenoxyethanol, cetyl alcohol, caprylyl glycol, 1,2 hexanediol, triethanolamine, xanthan gum, trisodium ethylenediamine disuccinate, parfum, bisabolol, panax ginseng root extract, lycium barbarum fruit extract, aloe barbadensis leaf juice, camellia oleifera leaf extract, butylene glycol, allantoin, tocopheryl acetate, limonene, butylphenyl methylpropional
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ADVANCED REVITAL
avobenzone, homosalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 54473-277 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.9 g in 50 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 3 g in 50 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 0.93 g in 50 mL Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CARNOSINE (UNII: 8HO6PVN24W) BETASIZOFIRAN (UNII: 2X51AD1X3T) ZINC PIDOLATE (UNII: C32PQ86DH4) SAPINDUS MUKOROSSI FRUIT RIND (UNII: 3D1P12PN9U) NIACINAMIDE (UNII: 25X51I8RD4) CALCIUM PANTOTHENATE (UNII: 568ET80C3D) TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) UBIDECARENONE (UNII: EJ27X76M46) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LEVOMENOL (UNII: 24WE03BX2T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TETRAPEPTIDE-21 (UNII: 179JUC43HU) ALLANTOIN (UNII: 344S277G0Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ASIAN GINSENG (UNII: CUQ3A77YXI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) ISOSORBIDE DICAPRYLATE (UNII: 0IK29C4889) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) AMINOPROPYL ASCORBYL PHOSPHATE (UNII: 290O2PQ83R) C12-16 ALCOHOLS (UNII: S4827SZE3L) PALMITIC ACID (UNII: 2V16EO95H1) TREHALOSE (UNII: B8WCK70T7I) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) SQUALANE (UNII: GW89575KF9) LYCIUM BARBARUM FRUIT (UNII: 930626MWDL) PHENOXYETHANOL (UNII: HIE492ZZ3T) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) SHEA BUTTER (UNII: K49155WL9Y) TRISILOXANE (UNII: 9G1ZW13R0G) MALTODEXTRIN (UNII: 7CVR7L4A2D) PROPANEDIOL (UNII: 5965N8W85T) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) PULLULAN (UNII: 8ZQ0AYU1TT) MONTMORILLONITE (UNII: A585MN1H2L) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) CITRUS BIOFLAVONOIDS (UNII: BD70459I50) BROCCOLI SPROUT (UNII: 128UH9LOAE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54473-277-01 1 in 1 BOX 01/01/2018 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/01/2017 Labeler - Melaleuca, Inc. (139760102) Establishment Name Address ID/FEI Business Operations Melaleuca, Inc. 079711683 manufacture(54473-277)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.