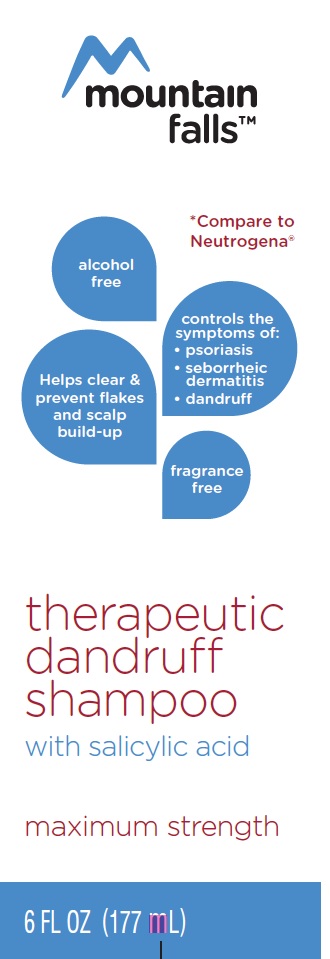
THERAPEUTIC DANDRUFF- dandruff shampoo shampoo
Therapeutic Dandruff by
Drug Labeling and Warnings
Therapeutic Dandruff by is a Otc medication manufactured, distributed, or labeled by Vi-Jon. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- claims
- Active ingredient
- Purpose
- Use
- Warnings
- Ask a doctor before use if you have
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
- Inactive ingredients
- Disclaimer
- Adverse Reactions Section
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THERAPEUTIC DANDRUFF
dandruff shampoo shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 11344-975 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) SODIUM C14 OLEFIN SULFONATE (UNII: N816E2SOKI) LINOLEAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: 5Q87K461JO) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) POLYQUATERNIUM-22 (4500 MPA.S) (UNII: H3W1D31JAR) HEXYLENE GLYCOL (UNII: KEH0A3F75J) SODIUM CITRATE (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11344-975-30 1 in 1 BOX 08/01/2017 1 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 08/01/2017 Labeler - Vi-Jon (150931459) Registrant - Vi-Jon (790752542) Establishment Name Address ID/FEI Business Operations Vi-Jon 088520668 manufacture(11344-975)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.