Hua Jing, Alcohol Pad, Alcohol, Thin 5050, Thick 9070
Alcohol Pad by
Drug Labeling and Warnings
Alcohol Pad by is a Otc medication manufactured, distributed, or labeled by Hua Jing Medical Supplies INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
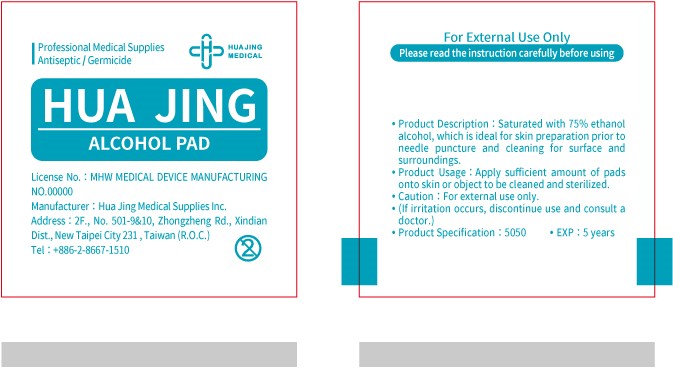
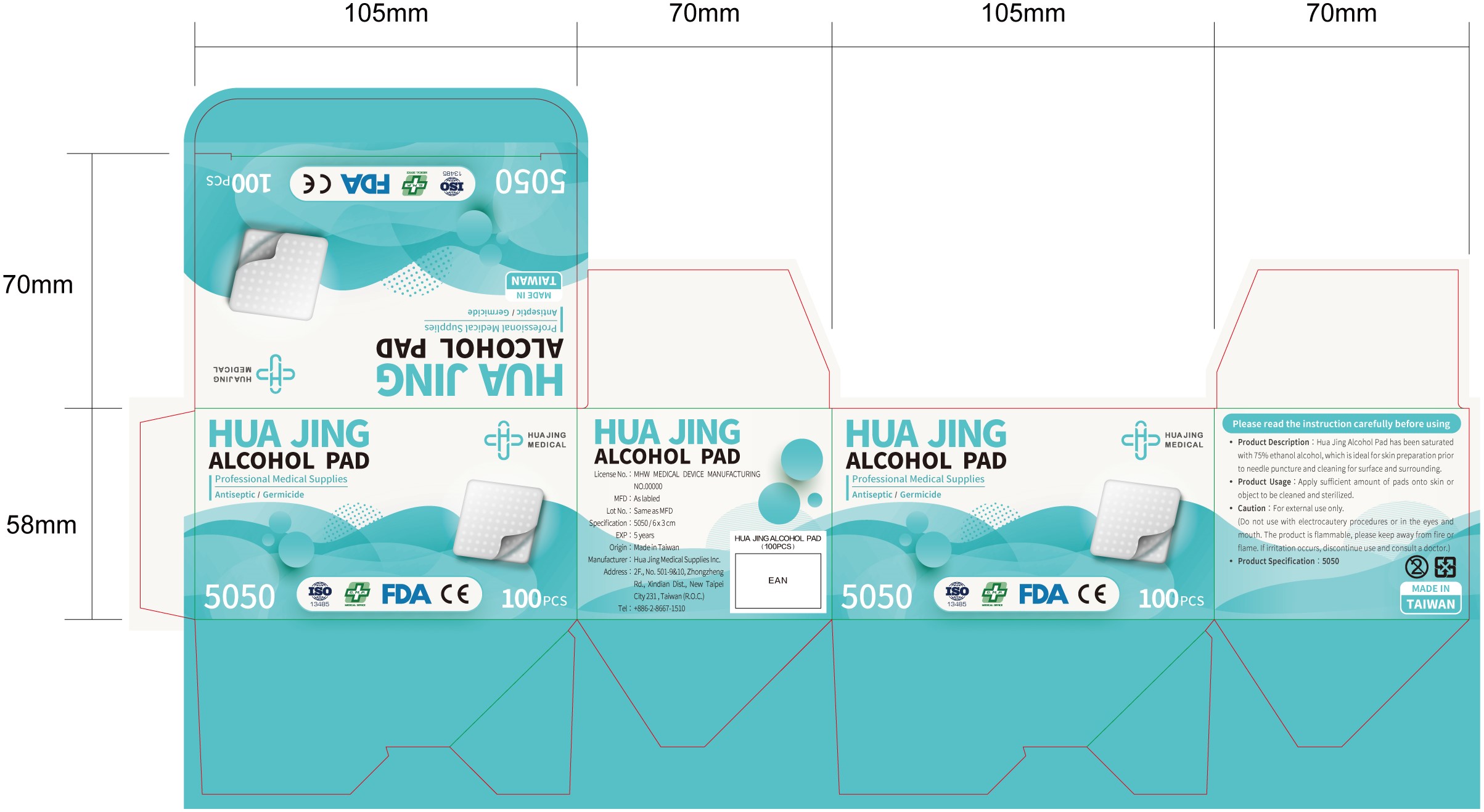
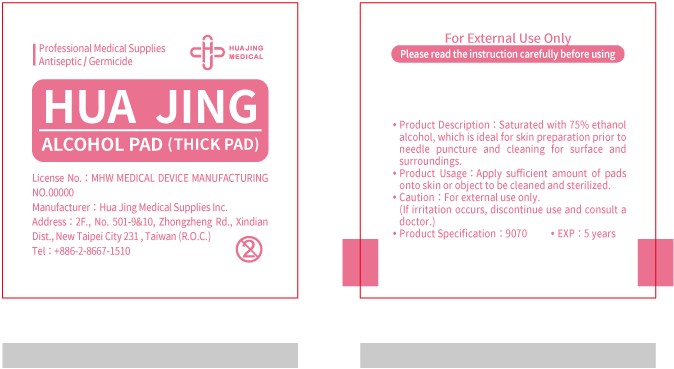
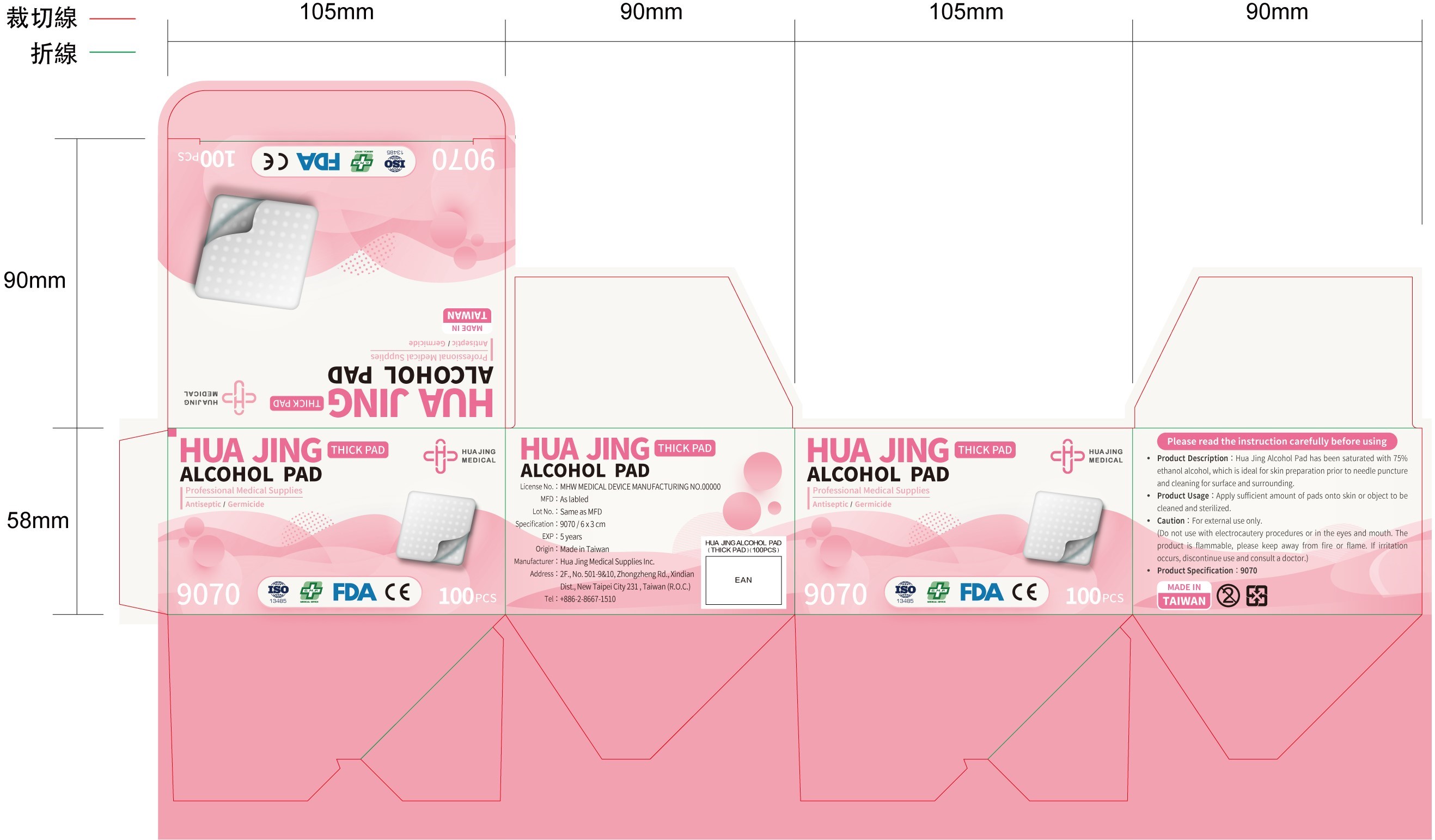
ALCOHOL PAD- alcohol cloth
Hua Jing Medical Supplies INC.
----------
Hua Jing, Alcohol Pad, Alcohol, Thin 5050, Thick 9070
Product Description
Hua Jing Alcohol Pad has been saturated with 75% ethanol alcohol, which is ideal for skin preparation prior to needle puncture and cleaning for surface and surrounding.
| ALCOHOL PAD
alcohol cloth |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Hua Jing Medical Supplies INC. (657582729) |
| Registrant - Hua Jing Medical Supplies INC. (657582729) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hua Jing Medical Supplies INC. | 657582729 | manufacture(83170-101) | |
Revised: 12/2024
Document Id: 2983e486-930c-7609-e063-6294a90a325f
Set id: f019e30b-93eb-22a4-e053-2a95a90a4180
Version: 4
Effective Time: 20241217