CLARITIN-D 24 HOUR- loratadine and pseudoephedrine sulfate tablet, extended release
Claritin-D by
Drug Labeling and Warnings
Claritin-D by is a Otc medication manufactured, distributed, or labeled by Bayer HealthCare LLC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- sneezing
- itchy, watery eyes
- runny nose
- itching of the nose or throat
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- reduces swelling of nasal passages
- temporarily relieves sinus congestion and pressure
- temporarily restores freer breathing through the nose
-
Warnings
Do not use
- if you have ever had an allergic reaction to this product or any of its ingredients
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- thyroid disease
- high blood pressure
- diabetes
- trouble urinating due to an enlarged prostate gland
- liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
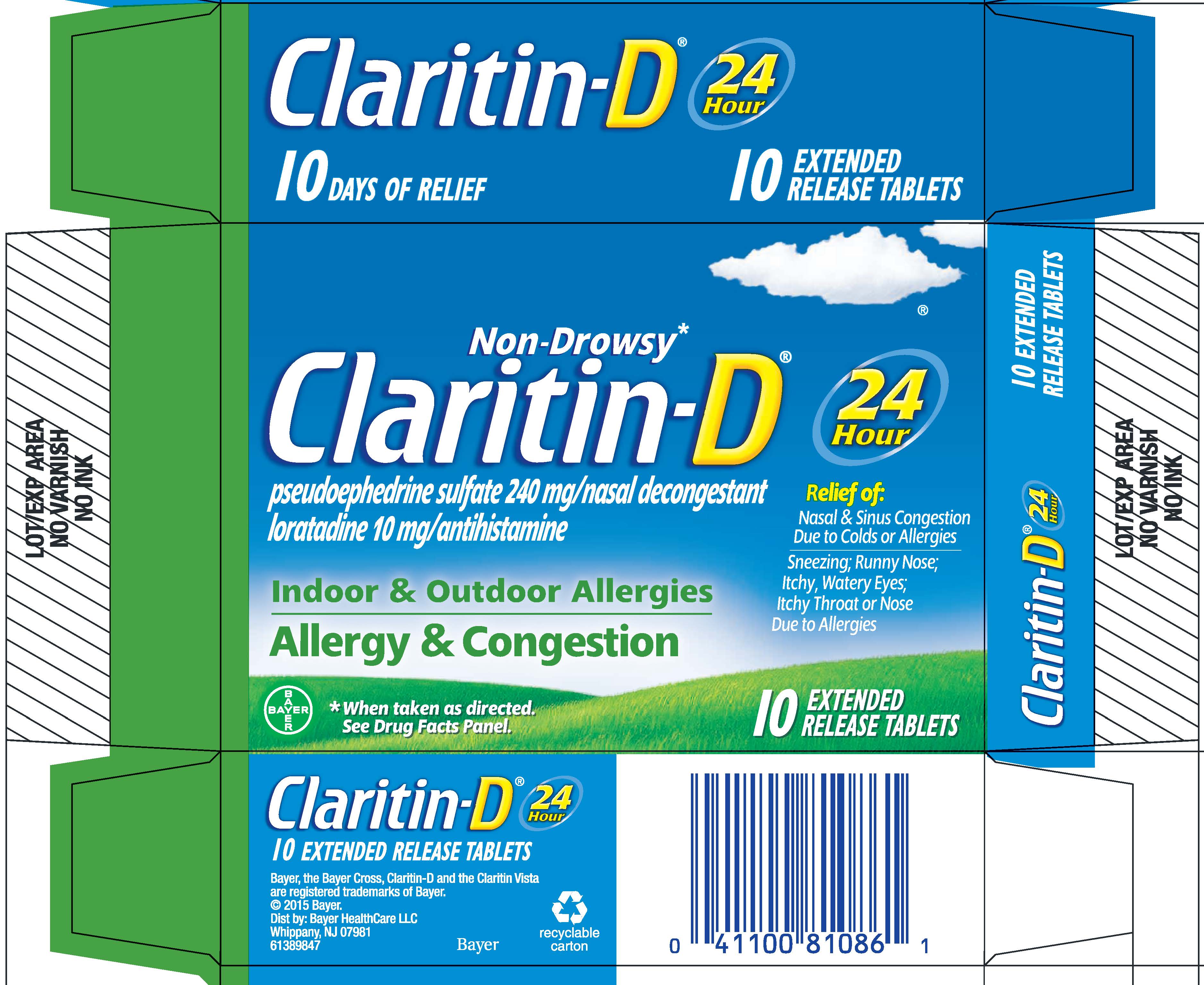
PRINCIPAL DISPLAY PANEL - 10 Tablet Blister Pack Carton
NDC: 11523-4332-1
Non-Drowsy*
Claritin-D ®
pseudoephedrine sulfate 240 mg/nasal decongestant
loratadine 10 mg/antihistamineIndoor & Outdoor Allergies
Allergy & Congestion24
HourRelief of:
Nasal & Sinus Congestion
Due to Colds or Allergies
Sneezing; Runny Nose;
Itchy, Watery Eyes;
Itchy Throat or Nose
Due to Allergies* When taken as directed. See Drug Facts Panel.
5
EXTENDED
RELEASE TABLETS
-
INGREDIENTS AND APPEARANCE
CLARITIN-D 24 HOUR
loratadine and pseudoephedrine sulfate tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 11523-4332 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg PSEUDOEPHEDRINE SULFATE (UNII: Y9DL7QPE6B) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE SULFATE 240 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSES (UNII: 3NXW29V3WO) ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SUCROSE (UNII: C151H8M554) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (white to off-white) Score no score Shape ROUND Size 18mm Flavor Imprint Code Claritin;D;24 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11523-4332-1 1 in 1 CARTON 12/01/2009 1 5 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 11523-4332-2 1 in 1 CARTON 12/01/2009 2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC: 11523-4332-3 3 in 1 CARTON 12/01/2009 3 5 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020470 12/01/2009 Labeler - Bayer HealthCare LLC. (112117283)
Trademark Results [Claritin-D]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CLARITIN-D 76506212 2819388 Live/Registered |
BAYER HEALTHCARE LLC 2003-04-14 |
 CLARITIN-D 74659413 1960278 Dead/Cancelled |
Schering Corporation 1995-04-05 |
 CLARITIN-D 74475937 1912214 Live/Registered |
BAYER HEALTHCARE LLC 1994-01-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.