PANACUR- fenbendazole paste
Panacur by
Drug Labeling and Warnings
Panacur by is a Animal medication manufactured, distributed, or labeled by Schering Corporation, Intervet Production S.A., Zhejiang Apeloa Kangyu Pharmaceutical Co., Ltd, Intervet Mexico S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
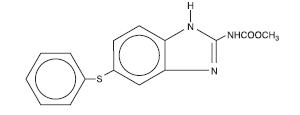
Panacur® (fenbendazole) Paste 10% contains the active anthelmintic, fenbendazole. The chemical name of fenbendazole is methyl 5-(phenylthio)-2-benzimidazole carbamate.
The chemical structure is:
Each gram of Panacur® (fenbendazole) Paste 10% contains 100 mg of fenbendazole and is flavored with artificial apple-cinnamon liquid.
- ACTIONS
-
INDICATIONS
Panacur® (fenbendazole) Paste 10% is indicated for the control of large strongyles (Strongylus edentatus, S. equinus, S. vulgaris), encysted early third stage (hypobiotic), late third stage and fourth stage cyathostome larvae, small strongyles, pinworms (Oxyuris equi), ascarids (Parascaris equorum), and arteritis caused by fourth stage larvae of Strongylus vulgaris in horses.
Panacur® (fenbendazole) Paste 10% is approved for use concomitantly with an approved form of trichlorfon. Trichlorfon is approved for the treatment of stomach bots (Gasterophilus spp.) in horses. Refer to the manufacturer's label for directions for use and cautions for trichlorfon.
-
PRECAUTIONS
Side effects associated with Panacur® (fenbendazole) Paste 10% could not be established in well-controlled safety studies in horses with single doses as high as 454 mg/lb (1,000 mg/kg) and 15 consecutive daily doses of 22.7 mg/lb (50 mg/kg). Particularly with higher doses, the lethal action of fenbendazole may cause the release of antigens by the dying parasites. This phenomenon may result in either a local or systemic hypersensitive reaction. As with any drug, these reactions should be treated symptomatically.
Panacur® (fenbendazole) Paste 10% has been evaluated for safety in pregnant mares during all stages of gestation with doses as high as 11.4 mg/lb (25 mg/kg) and in stallions with doses as high as 11.4 mg/lb (25 mg/kg). No adverse effects on reproductivity were detected. The recommended dose for control of 4th stage larvae of Strongylus vulgaris, 4.6 mg/lb (10 mg/kg) daily for 5 consecutive days, has not been evaluated for safety in stallions or pregnant mares.
Internal Parasites
Regular deworming at intervals of six to eight weeks may be required due to the possibility of reinfection.
Migrating Tissue Parasites
In the case of 4th stage larvae of Strongylus vulgaris, treatment and retreatment should be based on the life cycle and the epidemiology. Treatment should be initiated in the spring and repeated in the fall after a six month interval.
Optimum Deworming Program for control of S. vulgaris
Optimum reduction of S. vulgaris infections is achieved by reducing the infectivity of the pastures. When horses are running on pasture, in temperate North America, maximum pasture infectivity occurs in October-December. If horses are removed from those pastures in January, pasture infectivity will decline to zero by July 1. Egg production of S. vulgaris is minimal from January through April, peaking in August and declining to minimal values in December.
Recommended Deworming Program
**December 1, February 1, April 1, June 1, August 1, October 1.
The two treatments that are in bold type are the recommended periods when the 5 day treatment regimen for the control of the migrating larvae of S. vulgaris should be performed.
**For other areas in the world, retreatment periods for the migrating larvae of S. vulgaris may be different; consult with your veterinarian.
- CAUTIONS
- WARNING
-
DOSAGE
Panacur® (fenbendazole) Paste 10% is administered orally at a rate of 2.3 mg/lb (5 mg/kg) for the control of large strongyles, small strongyles, and pinworms. One syringe will deworm a 1,100 lb horse. For foals and weanlings (less than 18 months of age) where ascarids are a common problem, the recommended dose is 4.6 mg/lb (10 mg/kg); one syringe will deworm a 550 lb horse.
For control of encysted early third stage (hypobiotic), late third stage and fourth stage cyathostome larvae, and fourth stage larvae of Strongylus vulgaris, the recommended dose is 4.6 mg/lb (10 mg/kg) daily for 5 consecutive days; administer one syringe for each 550 Ibs body weight per day.
SEE PRECAUTIONS FOR RETREATMENT RECOMMENDATIONS.
-
DIRECTIONS FOR USE
- Determine the weight of the horse.
- Remove syringe tip.
- Turn the dial ring until the edge of the ring nearest the tip lines up with zero.
- Depress plunger to advance paste to tip.
- Now set the dial ring at the graduation nearest the weight of the horse (do not underdose).
- Horse's mouth must be free of food.
- Insert nozzle of syringe through the interdental space and deposit the paste on the back of the tongue by depressing the plunger.
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
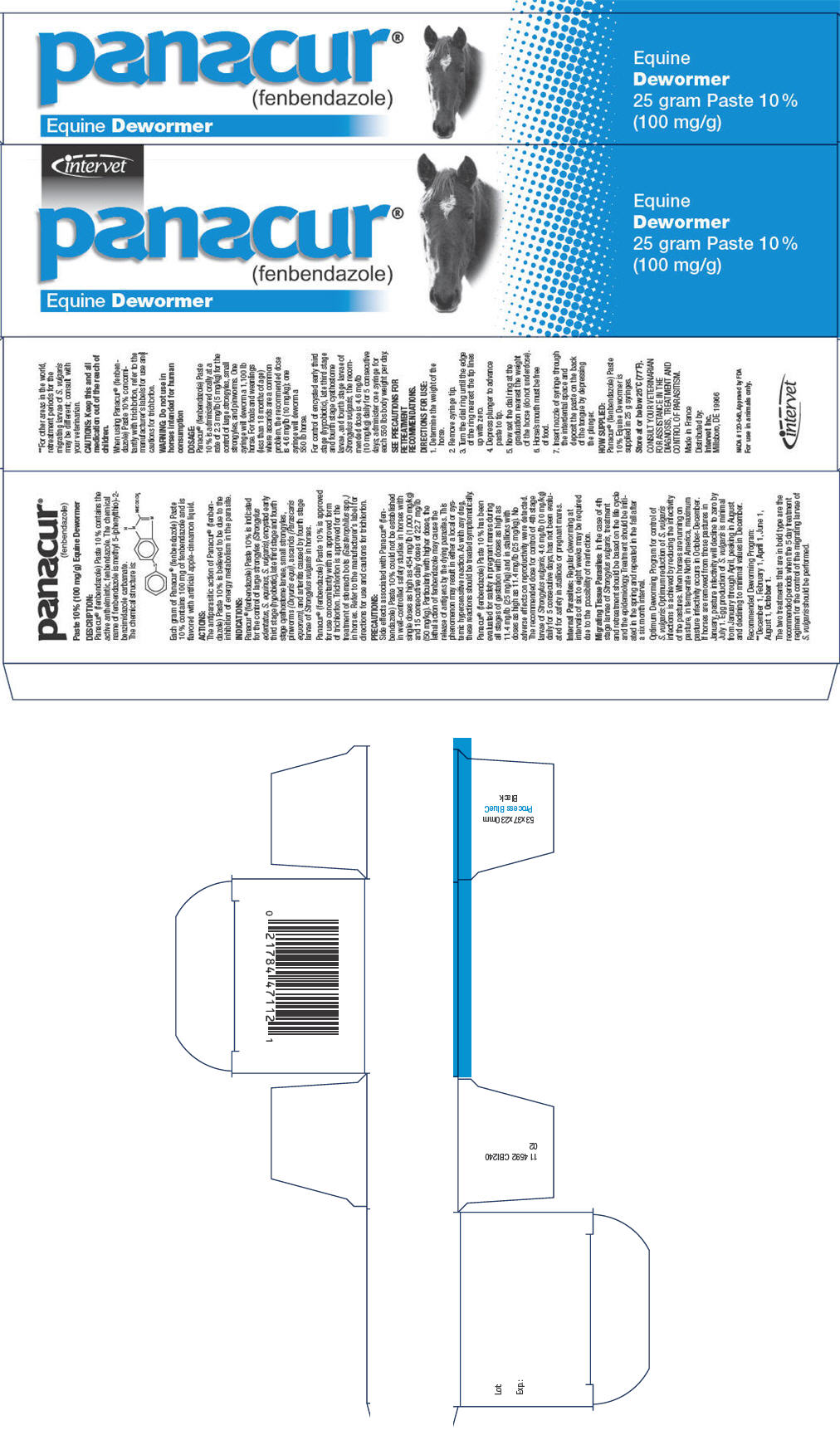
- PRINCIPAL DISPLAY PANEL - 100 mg/g Syringe Carton
-
PRINCIPAL DISPLAY PANEL - 100 mg/g Syringe Label
intervet
panacur®
(fenbendazole)Equine Dewormer 57 gram Paste 10% (100 mg/g)
FOR USE IN ANIMALS ONLY.
Net Wt. 57 g (2.01 oz)WARNING: DO NOT USE IN HORSES INTENDED FOR HUMAN CONSUMPTION.
DIRECTIONS:
- Determine the weight of the horse.
- Remove syringe tip.
- Turn the dial ring until the edge of the ring nearest the tip lines up with zero.
- Depress plunger to advance paste to tip.
- Now set the dial ring at the graduation nearest the weight of the horse
(do not underdose). - Horse's mouth should be free of food.
- Insert nozzle of syringe through the interdental space and deposit the paste on
the back of the tongue by depressing the plunger.
The contents of one syringe will deworm two 1,250 lb (568 kg) horses at the
standard dosage rate of 2.3 mg/lb (5 mg/kg). Refer to the carton for dosage and
full directions for use and for treatment of ascarids, encysted early third stage
(hypobiotic), late third stage and fourth stage cyathostome larvae and 4th stage
larvae of Strongylus vulgaris as well as for concomitant use with trichlorfon.
CONSULT YOUR VETERINARIAN FOR ASSISTANCE IN THE DIAGNOSIS,
TREATMENT AND CONTROL OF PARASITISM.
Each syringe contains 5.7 g of fenbendazole.Keep this and all medication out of the reach of children.
Store at or below 25°C (77°F).Manufactured by: DPT Laboratories,
San Antonio, TX 78215Panacur is the property of Intervet International
B.V. or affiliated companies or licensors and is
protected by copyrights, trademark and other
intellectual property laws. Copyright © 2011
Intervet International B.V. All rights reserved.
NADA # 120-648, Approved by FDA 129670UPC CODE
021784047159
-
INGREDIENTS AND APPEARANCE
PANACUR
fenbendazole pasteProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC: 57926-081 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fenbendazole (UNII: 621BVT9M36) (Fenbendazole - UNII:621BVT9M36) Fenbendazole 100 mg in 1 g Inactive Ingredients Ingredient Name Strength Carbomer Homopolymer Type B (Allyl Pentaerythritol Crosslinked) (UNII: HHT01ZNK31) Propylene Glycol (UNII: 6DC9Q167V3) Glycerin (UNII: PDC6A3C0OX) Sorbitol (UNII: 506T60A25R) Water (UNII: 059QF0KO0R) Sodium Hydroxide (UNII: 55X04QC32I) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Product Characteristics Color Score Shape Size Flavor APPLE, CINNAMON Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57926-081-44 1 in 1 CARTON 1 25 g in 1 SYRINGE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA120648 05/10/2010 PANACUR
fenbendazole pasteProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC: 57926-082 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fenbendazole (UNII: 621BVT9M36) (Fenbendazole - UNII:621BVT9M36) Fenbendazole 100 mg in 1 g Inactive Ingredients Ingredient Name Strength Carbomer Homopolymer Type B (Allyl Pentaerythritol Crosslinked) (UNII: HHT01ZNK31) Propylene Glycol (UNII: 6DC9Q167V3) Glycerin (UNII: PDC6A3C0OX) Sorbitol (UNII: 506T60A25R) Water (UNII: 059QF0KO0R) Sodium Hydroxide (UNII: 55X04QC32I) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Product Characteristics Color Score Shape Size Flavor APPLE, CINNAMON Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57926-082-48 1 in 1 CARTON 1 57 g in 1 SYRINGE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA120648 07/22/2011 Labeler - Schering Corporation (001317601) Establishment Name Address ID/FEI Business Operations Intervet Production S.A. 771867553 MANUFACTURE Establishment Name Address ID/FEI Business Operations DPT Laboratories, Ltd. 832224526 MANUFACTURE Establishment Name Address ID/FEI Business Operations Intervet Mexico S.A. de C.V. 588215863 API MANUFACTURE
Trademark Results [Panacur]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PANACUR 81039083 1039083 Dead/Cancelled |
Hoechst Aktiengesellschaft 0000-00-00 |
 PANACUR 73141362 1107717 Live/Registered |
HOECHST AKTIENGESELLSCHAFT 1977-09-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.