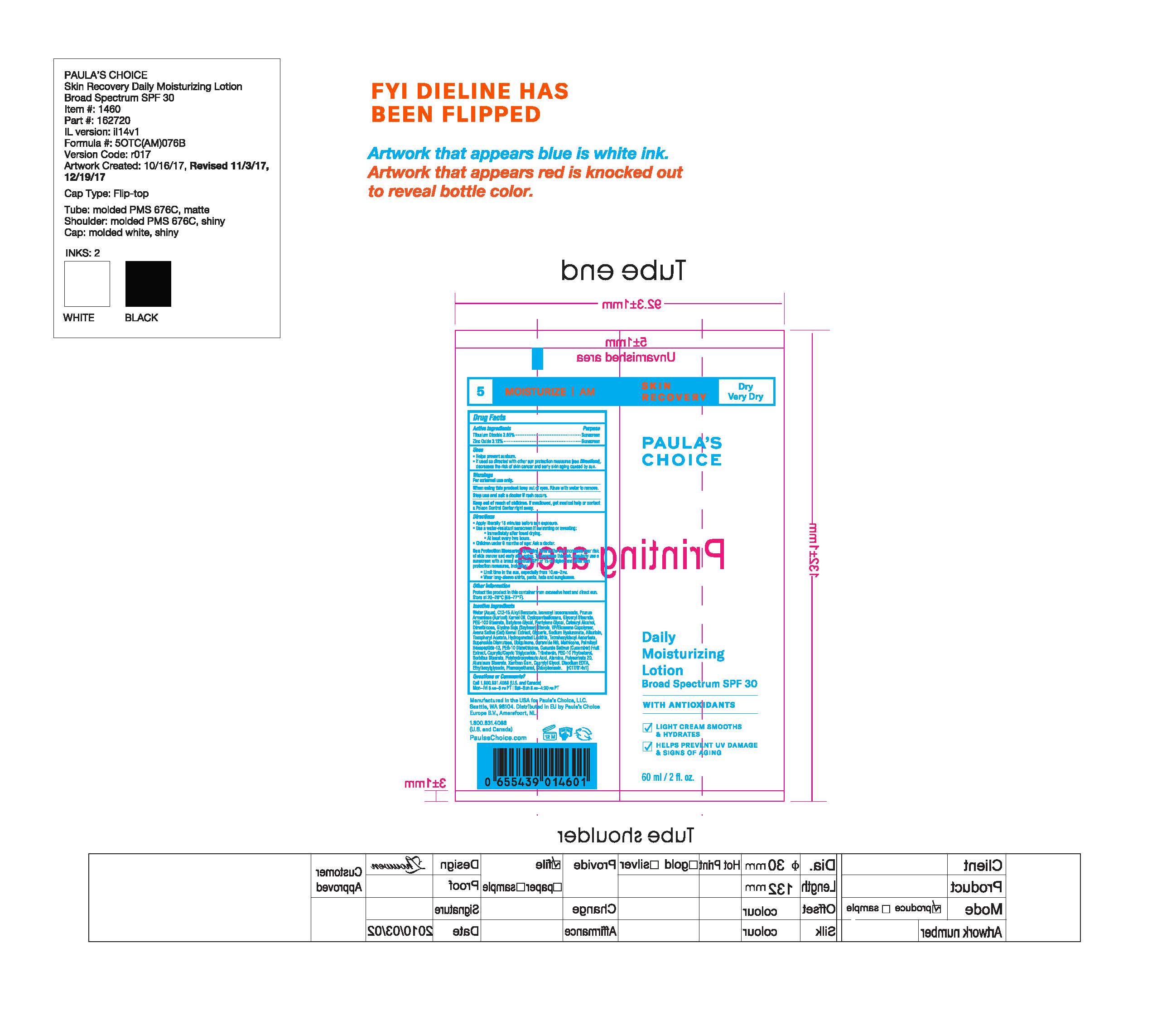
1460- Paulas Choice Skin Recovery Daily Moisturizing Lotion Broad Spectrum 30
Paulas Choice Skin Recovery Daily Moisturizing SPF 30 by
Drug Labeling and Warnings
Paulas Choice Skin Recovery Daily Moisturizing SPF 30 by is a Otc medication manufactured, distributed, or labeled by Paula's Choice LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PAULAS CHOICE SKIN RECOVERY DAILY MOISTURIZING SPF 30- titanium dioxide, zinc oxide lotion
Paula's Choice LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
1460- Paulas Choice Skin Recovery Daily Moisturizing Lotion Broad Spectrum 30
Directions for use:
Apply liberally 15 minuted before sun exposure
Use a water- resistant sunscreen if swimming or sweating:
- immediatly after towel drying
-At least every two hours
Children under 6 months of age: Ask a doctor
For external use only
When using this product keep out eyes. Rinse with water to remove. Stop use and ask a doctor if rash occurs.
Keep out of reach of children.
If swallowed,get medical help or contact a Poison Control Center right away.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask doctor if rash occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Water (Aqua), C12-15 Alkyl Benzoate, Isononyl Isononanoate, Prunus Armeniaca (Apricot) Kernel Oil, Cyclopentasiloxane, Glyceryl Stearate, PEG-100 Stearate, Butylene Glycol, Pentylene Glycol, Cetearyl Alcohol, Dimethicone, Glycine Soja (Soybean) Sterols, VP/Eicosene Copolymer, Avena Sativa (Oat) Kernel Extract, Glycerin, Sodium Hyaluronate, Allantoin, Tocopheryl Acetate, Hydrogenated Lecithin, Tetrahexyldecyl Ascorbate, Superoxide Dismutase, Ubiquinone, Ceramide NG, Methicone, Palmitoyl Hexapeptide-12, PEG-10 Dimethicone, Cucumis Sativus (Cucumber) Fruit Extract, Caprylic/Capric Triglyceride, Tribehenin, PEG-10 Phytosterol, Sorbitan Stearate, Polyhydroxystearic Acid, Alumina, Polysorbate 20, Aluminum Stearate, Xanthan Gum, Caprylyl Glycol, Disodium EDTA, Ethylhexylglycerin, Phenoxyethanol, Chlorphenesin.
| PAULAS CHOICE SKIN RECOVERY DAILY MOISTURIZING SPF 30
titanium dioxide, zinc oxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Paula's Choice LLC (029583981) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.