ALLEGRA ALLERGY- fexofenadine hydrochloride tablet, coated
Allegra Allergy by
Drug Labeling and Warnings
Allegra Allergy by is a Otc medication manufactured, distributed, or labeled by Chattem, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
- do not take more than directed
- Directions
- Other information
-
Inactive ingredients
croscarmellose sodium, D&C red 28, D&C red 33, FD&C blue 1, gelatin, hydroxypropylcellulose, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, PEG-135, pharmaceutical ink, pregelatinized starch, titanium dioxide
Questions or comments?
call toll-free 1-800-633-1610 or www.allegra.com
The makers of Allegra® do not make store brand products. The trade dress of this Allegra® package is subject to trademark protection.
Dist. By: Chattem, Inc., a Sanofi Company, Chattanooga, TN 37409-0219 ©2014 - PRINCIPAL DISPLAY PANEL
-
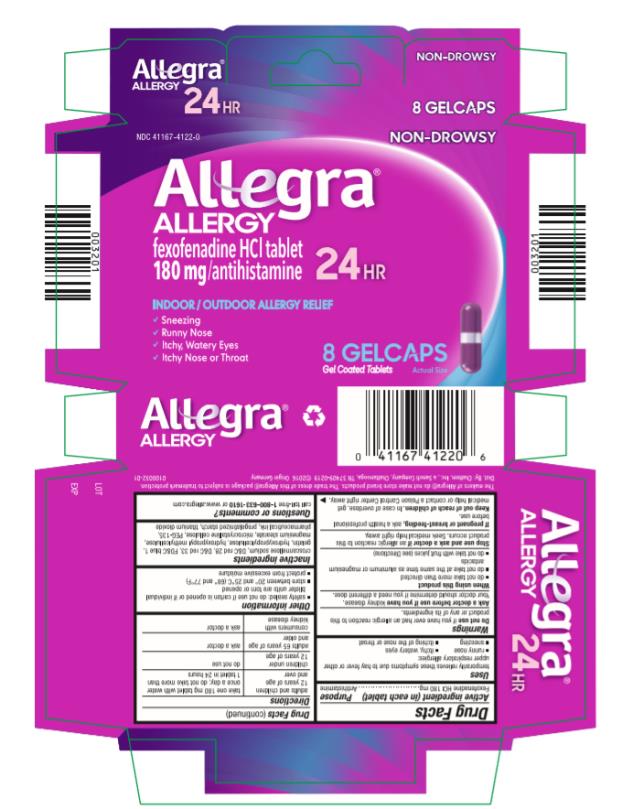
PRINCIPAL DISPLAY PANEL
NDC: 41167-4122-0
Allegra® Allergy
Fexofenadine HCI tablets
180 mg/antihistamine
24 HR
8 Gelcaps
PROFESSIONAL SAMPLE
NOT FOR RETAIL SALE
-
INGREDIENTS AND APPEARANCE
ALLEGRA ALLERGY
fexofenadine hydrochloride tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 41167-4122 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C RED NO. 28 (UNII: 767IP0Y5NH) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PURPLE (White band in the middle) Score no score Shape OVAL (Caplet) Size 20mm Flavor Imprint Code AG;AG Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 41167-4122-0 1 in 1 CARTON 12/17/2014 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 41167-4122-2 1 in 1 CARTON 12/17/2014 2 60 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC: 41167-4122-5 1 in 1 CARTON 12/17/2014 3 24 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC: 41167-4122-6 1 in 1 CARTON 12/17/2014 4 32 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC: 41167-4122-9 2 in 1 CARTON 12/17/2014 06/23/2019 5 NDC: 41167-4122-7 40 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020872 12/17/2014 Labeler - Chattem, Inc. (003336013)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.