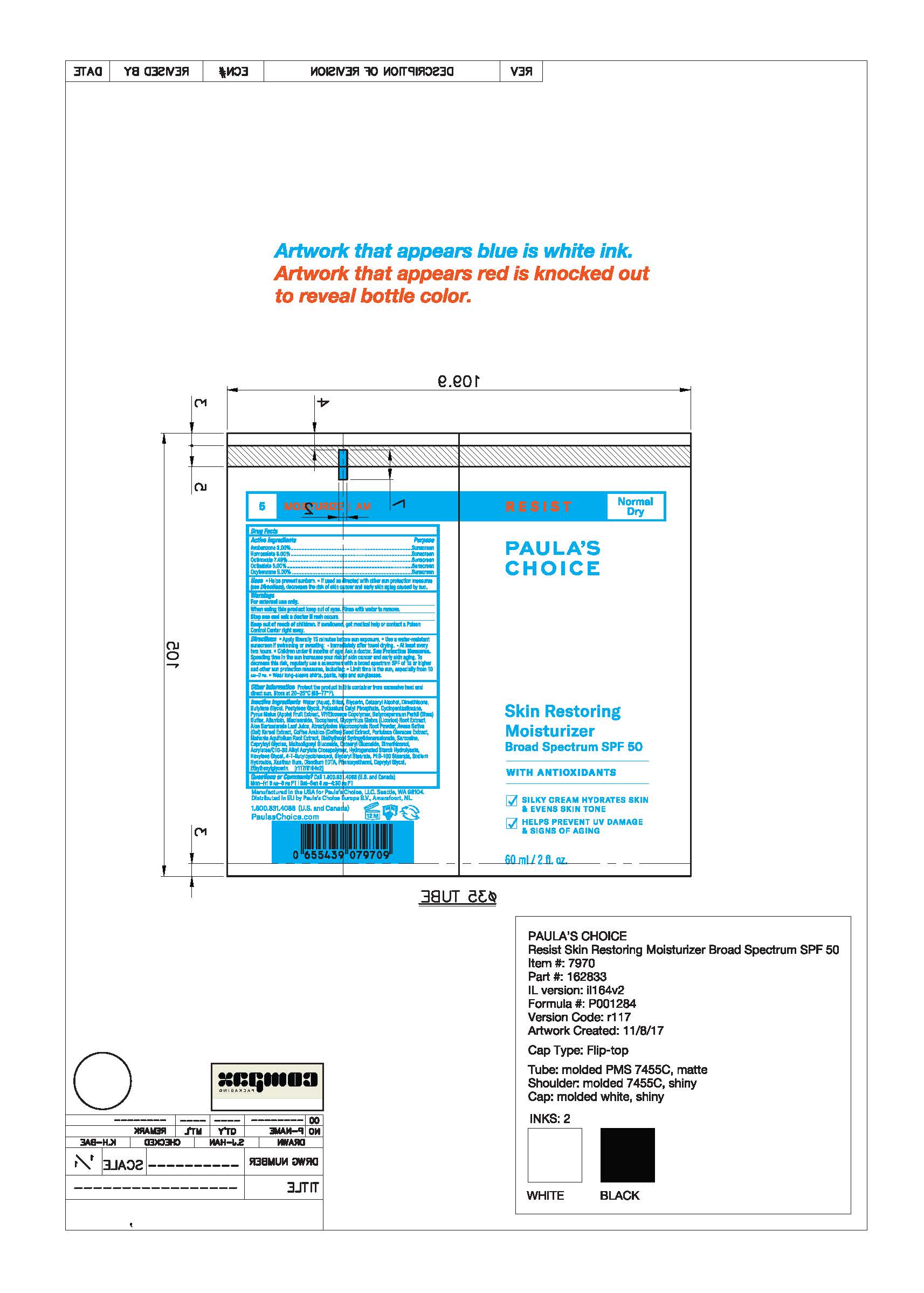
7970- RESIST Skin Restoring Moisturizer SPF 50
Paulas Choice RESIST Skin Restoring Moisturizer SPF 50 by
Drug Labeling and Warnings
Paulas Choice RESIST Skin Restoring Moisturizer SPF 50 by is a Otc medication manufactured, distributed, or labeled by Paula's Choice. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PAULAS CHOICE RESIST SKIN RESTORING MOISTURIZER SPF 50- avobenzone, homosalate,octinoxate, octisalate, oxybenzone lotion
Paula's Choice
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
7970- RESIST Skin Restoring Moisturizer SPF 50
Helps prevent sunburn
If used directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun.
Apply liberally 15 minuted before sun exposure
Use a water- resistant sunscreen if swimming or sweating:
- immediatly after towel drying
-At least every two hours
Children under 6 months of age: Ask a doctor
Sun Protection Measures.
Spending time in the su increases your risk of skin cancer and early skin aging.
To decrease this risk, regularly use a sunscreen with a broad spectrum SPF 15 or higher and other sun protection measures, including:
-Limit timw in the sun, especially from 10am-2pm
-Wear long-sleeve shirts, pants, hats, and sunglasses
Protect the product in this container from excessive heat and direct sun. Store at 20-25degrees Celcius.
Water (Aqua), Silica, Glycerin, Cetearyl Alcohol, Dimethicone, Butylene Glycol, Pentylene Glycol, Potassium Cetyl Phosphate, Cyclopentasiloxane, Pyrus Malus (Apple) Fruit Extract, VP/Eicosene Copolymer, Butyrospermum Parkii (Shea) Butter, Allantoin, Niacinamide, Tocopherol, Glycyrrhiza Glabra (Licorice) Root Extract, Aloe Barbadensis Leaf Juice, Atractylodes Macrocephala Root Powder, Avena Sativa (Oat) Kernel Extract, Coffea Arabica (Coffee) Seed Extract, Portulaca Oleracea Extract, Mahonia Aquifolium Root Extract, Diethylhexyl Syringylidenemalonate, Sarcosine, Capryloyl Glycine, Maltooligosyl Glucoside, Cetearyl Glucoside, Dimethiconol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Hydrogenated Starch Hydrolysate, Hexylene Glycol, 4-T-Butylcyclohexanol, Glyceryl Stearate, PEG-100 Stearate, Sodium Hydroxide, Xanthan Gum, Disodium EDTA, Phenoxyethanol, Caprylyl Glycol, Ethylhexylglycerin.
| PAULAS CHOICE RESIST SKIN RESTORING MOISTURIZER SPF 50
avobenzone, homosalate,octinoxate, octisalate, oxybenzone lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Paula's Choice (029583981) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.