Albuterol Sulfate Inhalation Solution, 1.25 mg/3 mL
Albuterol Sulfate Inhalation Solution by
Drug Labeling and Warnings
Albuterol Sulfate Inhalation Solution by is a Prescription medication manufactured, distributed, or labeled by The Ritedose Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ALBUTEROL SULFATE INHALATION SOLUTION- albuterol sulfate solution
The Ritedose Corporation
----------
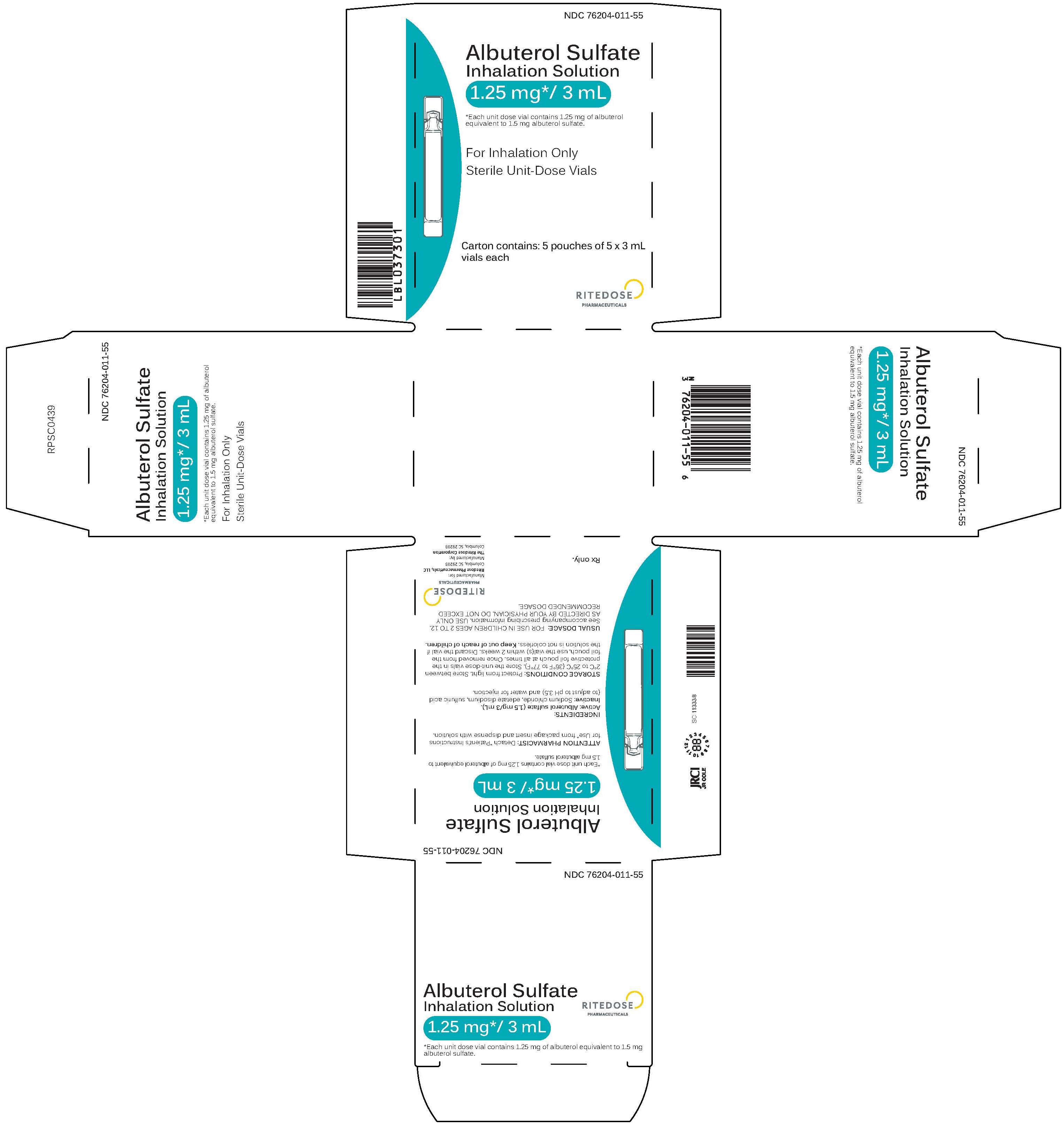
Albuterol Sulfate Inhalation Solution, 1.25 mg/3 mL
NDC: 76204-011-55
Albuterol Sulfate Inhalation Solution
1.25 mg* / 3 mL
*Each unit dose vial contains 1.25 mg of albuterol equivalent to 1.5 mg albuterol sulfate.
For Inhalation Only
Sterile Unit-Dose Vials
Carton contains: 5 pouches of 5 x 3 mL vials each.
| ALBUTEROL SULFATE INHALATION SOLUTION
albuterol sulfate solution |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| ALBUTEROL SULFATE INHALATION SOLUTION
albuterol sulfate solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - The Ritedose Corporation (837769546) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| The Ritedose Corporation | 837769546 | analysis(65302-061, 65302-065) , label(65302-061, 65302-065) , manufacture(65302-061, 65302-065) , pack(65302-061, 65302-065) | |
Revised: 12/2022
Document Id: f06d3ff4-0b82-bd33-e053-2a95a90af7e9
Set id: f06d3ff4-0b81-bd33-e053-2a95a90af7e9
Version: 1
Effective Time: 20221222