TOXEX by PEKANA Naturheilmittel GmbH TOXEX™
TOXEX by
Drug Labeling and Warnings
TOXEX by is a Homeopathic medication manufactured, distributed, or labeled by PEKANA Naturheilmittel GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
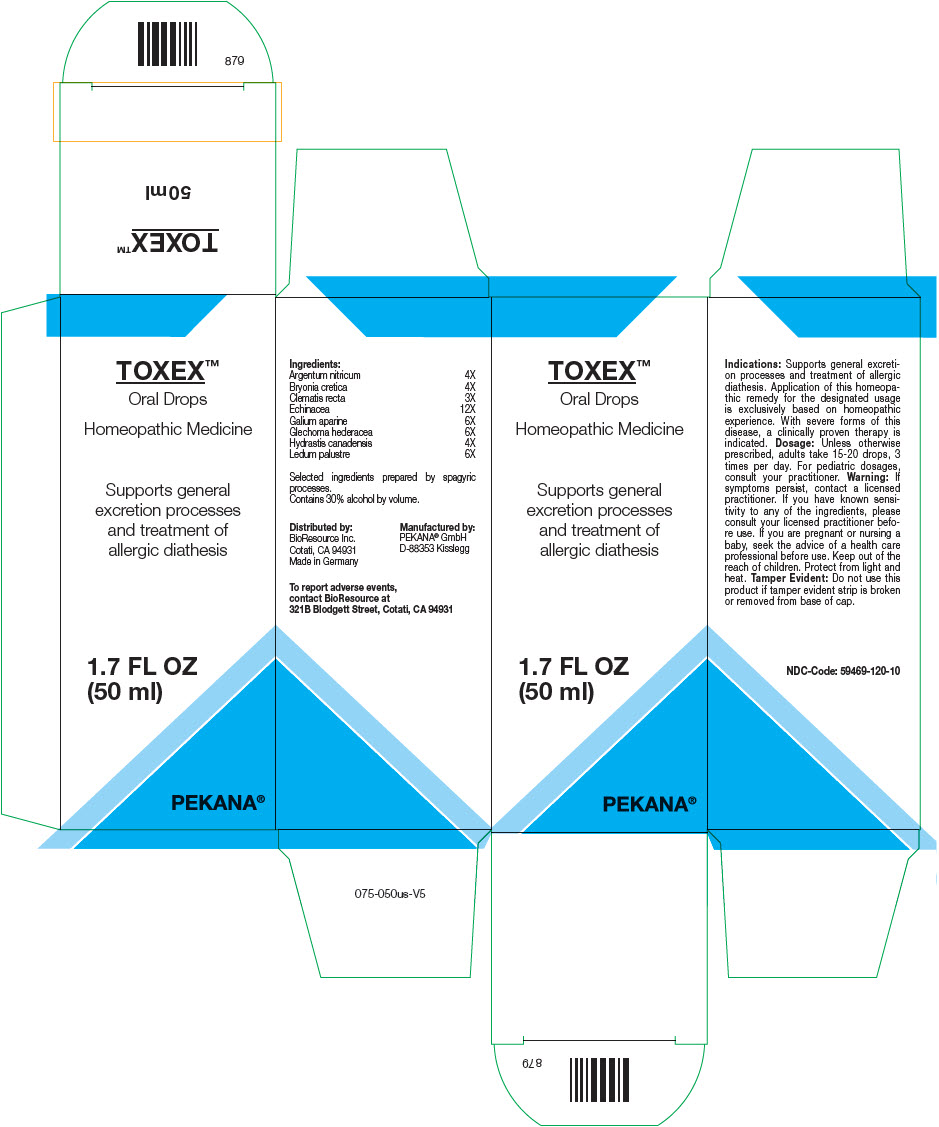
TOXEX- silver nitrate, bryonia dioica root, clematis recta flowering top, goldenseal, echinacea, unspecified, galium aparine, glechoma hederacea flowering top, and ledum palustre twig solution/ drops
PEKANA Naturheilmittel GmbH
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
TOXEX™
| Ingredients: | |
|---|---|
| Argentum nitricum | 4X |
| Bryonia cretica | 4X |
| Clematis recta | 3X |
| Echinacea | 12X |
| Galium aparine | 6X |
| Glechoma hederacea | 6X |
| Hydrastis canadensis | 4X |
| Ledum palustre | 6X |
Selected ingredients prepared by spagyric processes.
Dosage
Unless otherwise prescribed, adults take 15-20 drops, 3 times per day. For pediatric dosages, consult your practitioner.
| TOXEX
silver nitrate, bryonia dioica root, clematis recta flowering top, goldenseal, echinacea, unspecified, galium aparine, glechoma hederacea flowering top, and ledum palustre twig solution/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - PEKANA Naturheilmittel GmbH (320344542) |