LABETALOL HCL injection, solution
Labetalol HCl by
Drug Labeling and Warnings
Labetalol HCl by is a Prescription medication manufactured, distributed, or labeled by Cantrell Drug Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
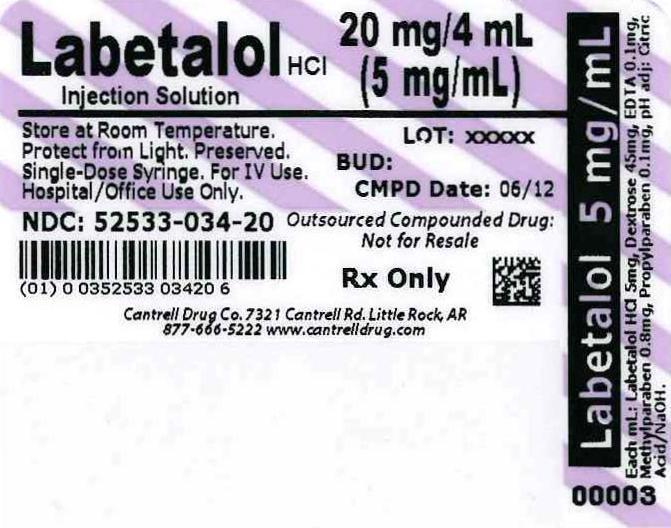
- Label
- SPL UNCLASSIFIED SECTION
-
INGREDIENTS AND APPEARANCE
LABETALOL HCL
labetalol hcl injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52533-034 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LABETALOL HYDROCHLORIDE (UNII: 1GEV3BAW9J) (LABETALOL - UNII:R5H8897N95) LABETALOL HYDROCHLORIDE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Anhydrous Dextrose (UNII: 5SL0G7R0OK) 45 mg in 1 mL Edetate Disodium (UNII: 7FLD91C86K) 0.1 mg in 1 mL Methylparaben (UNII: A2I8C7HI9T) 0.8 mg in 1 mL Propylparaben (UNII: Z8IX2SC1OH) 0.1 mg in 1 mL Citric Acid Monohydrate (UNII: 2968PHW8QP) Sodium Hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52533-034-20 4 mL in 1 SYRINGE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/23/2012 Labeler - Cantrell Drug Company (035545763)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.