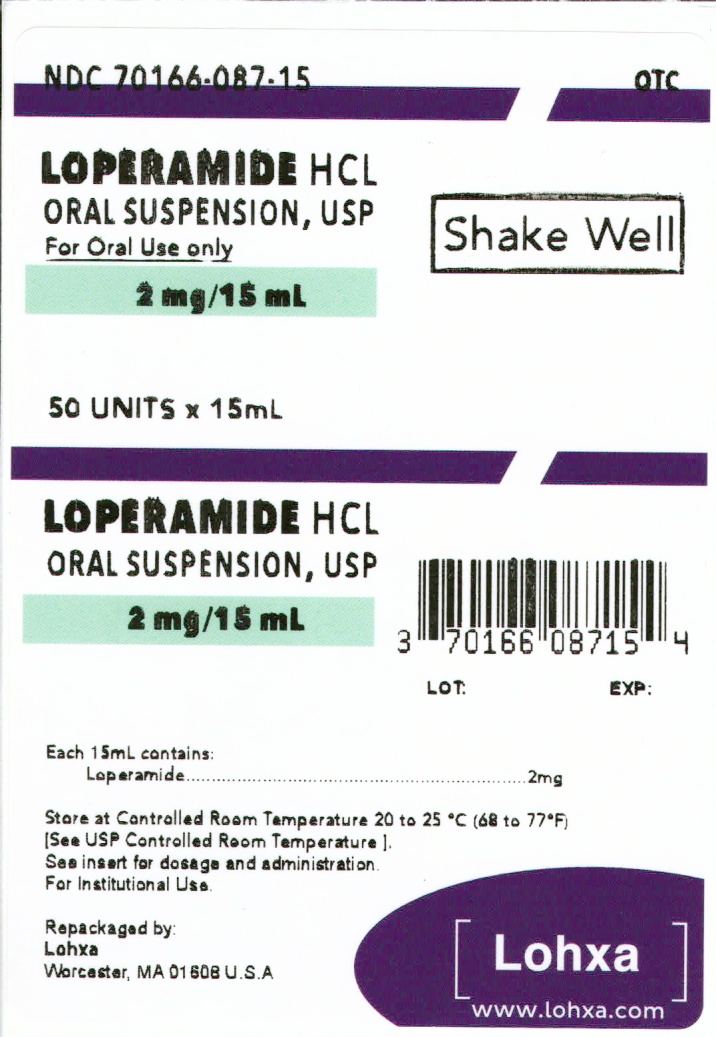
Loperamide Hydrochloride Oral Suspension
leader loperamide by
Drug Labeling and Warnings
leader loperamide by is a Otc medication manufactured, distributed, or labeled by Lohxa. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LEADER LOPERAMIDE- loperamide hydrochloride suspension
Lohxa
----------
Loperamide Hydrochloride Oral Suspension
Warnings
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCl
When using this product
- tiredness, drowsiness or dizziness may occur. Be careful when driving or operating machinery.
Stop use and ask a doctor if
- symptoms get worse
- diarrhea lasts for more than 2 days
- you get abdominal swelling or bulging.
These may be signs of a serious condition.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise use age.
- shake well before using
- only use attached measuring cup to dose product
|
adults and children 12 years and over |
30 mL (6 tsp) after the first loose stool; 15 mL (3 tsp) after each subsequent loose stool; but no more than 60 mL (12 tsp) in 24 hours |
|
children 9-11 years (60-95 lbs) |
15 mL (3 tsp) after the first loose stool; 7.5 mL (1 ½ tsp) after each subsequent loose stool; but no more than 45 mL (9 tsp) in 24 hours |
|
children 6-8 years (48-59 lbs) |
15 mL (3 tsp) after the first loose stool; 7.5 mL (1 ½ tsp) after each subsequent loose stool; but no more than 30 mL (6 tsp) in 24 hours |
|
children under 6 years (up to 47 lbs) |
ask a doctor |
Other information
- each 30 mL (6 tsp) contains: sodium 15 mg
- store between 20-25 °C (68-77 °F)
- see side panel for lot number and expiration date
| LEADER LOPERAMIDE
loperamide hydrochloride suspension |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Lohxa (079872715) |