EQUALINE FAST ACTING- phenylephrine hydrochloride spray
Equaline by
Drug Labeling and Warnings
Equaline by is a Otc medication manufactured, distributed, or labeled by SUPERVALU INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Uses
- Warnings
- Ask a doctor before use if you have
-
When using this product
do not exceed recommended dosage
do not use for more than 3 days. Use only as directed
temporary discomfort such as burning, stinging, sneezing, or an increase in nasal discharge may occur
the use of this container by more than one person may spread infection
frequent or prolonged use may cause nasal congestion to recur or worsen
- Stop use and ask a doctor if
- If pregnant or breast-feeding
- Keep out of reach of children
-
Directions
- adults and children 12 years of age and over: 2 or 3 sprays in each nostril not more than every 4 hours
- children under 12 years of age: ask a doctor
- Use instructions: with head in a normal, upright position, put atomizer tip into nostril. Squeeze bottle with firm, quick pressure while inhaling. Wipe nozzle clean after each use.
- Other information
- Inactive Ingredients
-
Questions
1-877-932-7948
IMPORTANT: Keep this carton for future reference on full labeling
DO NOT USE IF PRINTED SEAL OVER CAP IS TORN OR MISSING
Distributed By: Supervalu Inc.
Eden Prairie, MN 55344This product is not manufactured or distributed by Novartis Consumer Health Inc., the owner of the registered trademark 4-Way Fast Acting Nasal Spray.
-
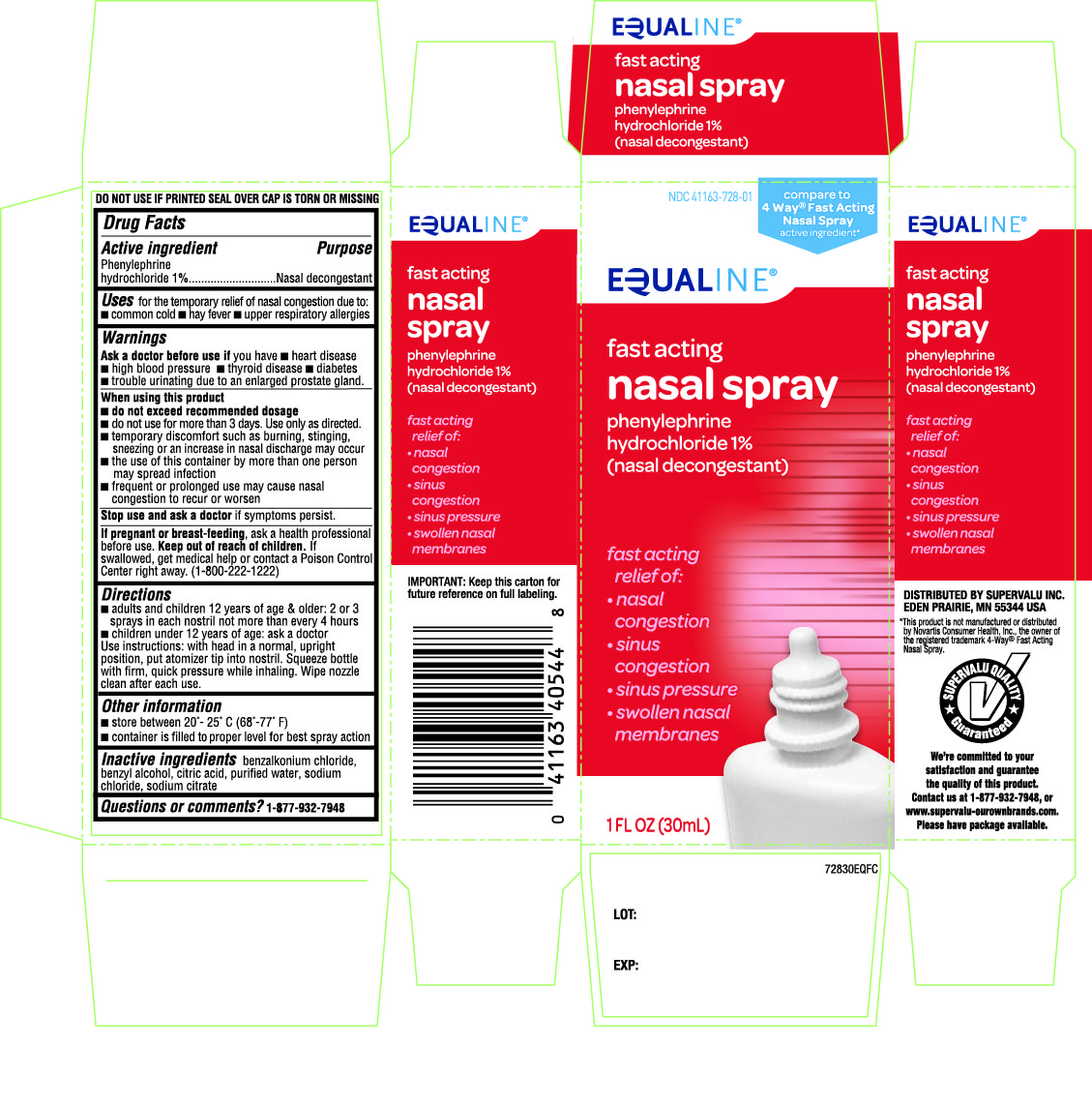
Principal Display
NDC: 41163-728-01
Equaline
Fast Acting
Nasal Spray
Phenylephrine hydrochloride 1%
(nasal decongestant)
fast acting relief of
- nasal congestion
- sinus congestion
- sinus pressure
- swollen nasal passages
1 FL OZ (30mL)
-
INGREDIENTS AND APPEARANCE
EQUALINE FAST ACTING
phenylephrine hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 41163-728 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BENZYL ALCOHOL (UNII: LKG8494WBH) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM CITRATE (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 41163-728-01 1 in 1 CARTON 1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 10/30/2014 Labeler - SUPERVALU INC. (006961411)
Trademark Results [Equaline]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EQUALINE 78379410 3177770 Live/Registered |
SUPERVALU LICENSING LLC 2004-03-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.