INREBIC- fedratinib hydrochloride capsule
Inrebic by
Drug Labeling and Warnings
Inrebic by is a Prescription medication manufactured, distributed, or labeled by Celgene Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use INREBIC safely and effectively. See full prescribing information for INREBIC.
INREBIC® (fedratinib) capsules, for oral use
Initial U.S. Approval: 2019WARNING: ENCEPHALOPATHY INCLUDING WERNICKE'S
See full prescribing information for complete boxed warning.
Serious and fatal encephalopathy, including Wernicke's, has occurred in patients treated with INREBIC. Wernicke's encephalopathy is a neurologic emergency. Assess thiamine levels in all patients prior to starting INREBIC, periodically during treatment, and as clinically indicated. Do not start INREBIC in patients with thiamine deficiency; replete thiamine prior to treatment initiation. If encephalopathy is suspected, immediately discontinue INREBIC and initiate parenteral thiamine. Monitor until symptoms resolve or improve and thiamine levels normalize. (2.6, 5.1, 6.1).
INDICATIONS AND USAGE
- INREBIC is a kinase inhibitor indicated for the treatment of adult patients with intermediate-2 or high-risk primary or secondary (post-polycythemia vera or post-essential thrombocythemia) myelofibrosis (MF) (1).
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Capsules: 100 mg (3).
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Anemia and Thrombocytopenia: Manage by dose reduction, interruption, or transfusion (5.2).
- Gastrointestinal Toxicity: Manage by dose reduction or interruption if patient develops severe diarrhea, nausea, or vomiting. Prophylaxis with anti-emetics and treatment with anti-diarrhea medications are recommended (5.3).
- Hepatic Toxicity: Manage by dose reduction or interruption (5.4).
- Amylase and Lipase Elevation: Manage by dose reduction or interruption (5.5).
ADVERSE REACTIONS
The most common adverse reactions (≥20%) are diarrhea, nausea, anemia, and vomiting (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Celgene Corporation at 1-888-423-5436 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: ENCEPHALOPATHY INCLUDING WERNICKE'S
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Monitoring for Safety
2.3 Dose Modifications with Concomitant Use of Strong CYP3A4 Inhibitors
2.4 Dose Modifications for Severe Renal Impairment
2.5 Dose Modifications for Adverse Reactions
2.6 Management of Thiamine Levels and Wernicke's Encephalopathy (WE)
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Encephalopathy, Including Wernicke's
5.2 Anemia and Thrombocytopenia
5.3 Gastrointestinal Toxicity
5.4 Hepatic Toxicity
5.5 Amylase and Lipase Elevation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on INREBIC
7.2 Effect of INREBIC on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: ENCEPHALOPATHY INCLUDING WERNICKE'S
Serious and fatal encephalopathy, including Wernicke's, has occurred in patients treated with INREBIC. Wernicke's encephalopathy is a neurologic emergency. Assess thiamine levels in all patients prior to starting INREBIC, periodically during treatment, and as clinically indicated. Do not start INREBIC in patients with thiamine deficiency; replete thiamine prior to treatment initiation. If encephalopathy is suspected, immediately discontinue INREBIC and initiate parenteral thiamine. Monitor until symptoms resolve or improve and thiamine levels normalize [see Dosage and Administration (2.6), Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Conduct baseline testing of thiamine (Vitamin B1) levels prior to initiation of INREBIC [see Dosage and Administration (2.2), Warnings and Precautions (5.1)].
The recommended dosage of INREBIC is 400 mg taken orally once daily for patients with a baseline platelet count of greater than or equal to 50 × 109/L.
INREBIC may be taken with or without food. Administration with a high fat meal may reduce the incidence of nausea and vomiting.
Modify the dose for patients using concomitant strong CYP3A4 inhibitors, and in patients with severe renal impairment (creatinine clearance (CLcr) 15 mL/min to 29 mL/min) [see Dosage and Administration (2.3, 2.4)].
If a dose of INREBIC is missed, the next scheduled dose should be taken the following day.
Patients that are on treatment with ruxolitinib before the initiation of INREBIC must taper and discontinue according to the ruxolitinib prescribing information.
2.2 Monitoring for Safety
Obtain the following blood tests prior to starting treatment with INREBIC, periodically during treatment, and as clinically indicated [see Warnings and Precautions (5.1, 5.2, 5.4, 5.5)]:
- Thiamine (Vitamin B1) level
- Complete blood count with platelets
- Creatinine and BUN
- Hepatic panel
- Amylase and lipase
2.3 Dose Modifications with Concomitant Use of Strong CYP3A4 Inhibitors
Reduce INREBIC dose when administering with strong CYP3A4 inhibitors to 200 mg once daily.
In cases where co-administration with a strong CYP3A4 inhibitor is discontinued, INREBIC dosage should be increased to 300 mg once daily during the first two weeks after discontinuation of the CYP3A4 inhibitor, and then to 400 mg once daily thereafter as tolerated [see Drug Interactions (7.1)].
2.4 Dose Modifications for Severe Renal Impairment
Reduce INREBIC dose to 200 mg once daily in patients with severe renal impairment (creatinine clearance (CLcr) 15 mL/min to 29 mL/min as estimated by Cockcroft-Gault (C-G) equation).
2.5 Dose Modifications for Adverse Reactions
Modify dose for hematologic and non-hematologic adverse reactions per Table 1 and Table 2. Discontinue INREBIC in patients unable to tolerate a dose of 200 mg daily. See Warnings and Precautions for other mitigating strategies.
Table 1: Dose Modifications for Hematologic Adverse Reactions Hematologic Adverse Reactions Dose Reduction Grade 4 Thrombocytopenia or
Grade 3 Thrombocytopenia with active bleedingInterrupt dose until resolved to Grade 2 or lower or baseline. Restart dose at 100 mg daily below the last given dose. Grade 4 Neutropenia Interrupt dose until resolved to Grade 2 or lower or baseline. Restart dose at 100 mg daily below the last given dose. Consider dose reductions for patients who become transfusion-dependent during treatment with INREBIC.
Table 2: Dose Reductions for Non-hematologic Adverse Reactions Non-hematologic Adverse Reactions Dose Reduction Grade 3 or higher Nausea, Vomiting, or Diarrhea not responding to supportive measures within 48 hours Interrupt dose until resolved to Grade 1 or lower or baseline. Restart dose at 100 mg daily below the last given dose. Grade 3 or higher ALT, AST, or Bilirubin Interrupt dose until resolved to Grade 1 or lower or baseline. Restart dose at 100 mg daily below the last given dose.
Monitor ALT, AST, and bilirubin (total and direct) more frequently following the dose reduction. If re-occurrence of a Grade 3 or higher elevation, discontinue treatment with INREBIC.Grade 3 or higher Other Non-hematologic Toxicities Interrupt dose until resolved to Grade 1 or lower or baseline. Restart dose at 100 mg daily below the last given dose. 2.6 Management of Thiamine Levels and Wernicke's Encephalopathy (WE)
Assess thiamine levels and nutritional status prior to starting INREBIC and periodically during treatment and as clinically indicated. Do not start INREBIC in patients with thiamine deficiency; replete thiamine prior to treatment initiation and during treatment if thiamine levels are low. If Wernicke's encephalopathy is suspected, immediately discontinue treatment with INREBIC and initiate parenteral thiamine treatment. Monitor until symptoms resolve or improve and thiamine levels normalize [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Encephalopathy, Including Wernicke's
Serious and fatal encephalopathy, including Wernicke's encephalopathy, has occurred in INREBIC-treated patients. Serious cases were reported in 1.3% (8/608) of patients treated with INREBIC in clinical trials and 0.16% (1/608) of cases were fatal.
Wernicke's encephalopathy is a neurologic emergency resulting from thiamine (Vitamin B1) deficiency. Signs and symptoms of Wernicke's encephalopathy may include ataxia, mental status changes, and ophthalmoplegia (e.g., nystagmus, diplopia). Any change in mental status, confusion, or memory impairment should raise concern for potential encephalopathy, including Wernicke's, and prompt a full evaluation including a neurologic examination, assessment of thiamine levels, and imaging. Assess thiamine levels in all patients prior to starting INREBIC, periodically during treatment, and as clinically indicated. Do not start INREBIC in patients with thiamine deficiency; replete thiamine prior to treatment initiation. If encephalopathy is suspected, immediately discontinue INREBIC and initiate parenteral thiamine. Monitor until symptoms resolve or improve and thiamine levels normalize [see Dosage and Administration (2.6) and Clinical Trials Experience (6.1)].
5.2 Anemia and Thrombocytopenia
Treatment with INREBIC can cause anemia and thrombocytopenia.
Anemia
New or worsening Grade 3 anemia occurred in 34% of INREBIC-treated patients. The median time to onset of the first Grade 3 anemia was approximately 2 months, with 75% of cases occurring within 3 months. Mean hemoglobin levels reached nadir after 12 to 16 weeks with partial recovery and stabilization after 16 weeks. Red blood cell transfusions were received by 51% of INREBIC-treated patients and permanent discontinuation of INREBIC occurred due to anemia in 1% of patients. Consider dose reduction for patients who become red blood cell transfusion dependent [see Dosage and Administration (2.5)].
Thrombocytopenia
New or worsening Grade ≥3 thrombocytopenia during the randomized treatment period occurred in 12% of INREBIC-treated patients. The median time to onset of the first Grade 3 thrombocytopenia was approximately 1 month; with 75% of cases occurring within 4 months. Platelet transfusions were received by 3.1% of INREBIC-treated patients. Permanent discontinuation of treatment due to thrombocytopenia and bleeding that required clinical intervention both occurred in 2.1% of INREBIC-treated patients.
Obtain a complete blood count (CBC) at baseline, periodically during treatment, and as clinically indicated. For Grade 3 thrombocytopenia with active bleeding or Grade 4 thrombocytopenia, interrupt INREBIC until resolved to less than or equal to Grade 2 or baseline. Restart dose at 100 mg daily below the last given dose and monitor platelets as clinically indicated [see Dosage and Administration (2.5)].
5.3 Gastrointestinal Toxicity
Gastrointestinal toxicities are the most frequent adverse reactions in INREBIC-treated patients. During the randomized treatment period, diarrhea occurred in 66% of patients, nausea in 62% of patients, and vomiting in 39% of patients. Grade 3 diarrhea and vomiting occurred in 5% and 3.1% of patients, respectively. The median time to onset of any grade nausea, vomiting, and diarrhea was 1 day, with 75% of cases occurring within 2 weeks of treatment.
Consider providing appropriate prophylactic anti-emetic therapy (e.g., 5-HT3 receptor antagonists) during INREBIC treatment. Treat diarrhea with anti-diarrheal medications promptly at the first onset of symptoms. For Grade 3 or higher nausea, vomiting, or diarrhea not responsive to supportive measures within 48 hours, interrupt INREBIC until resolved to Grade 1 or less or baseline. Restart dose at 100 mg daily below the last given dose [see Dosage and Administration (2.5)]. Monitor thiamine levels and replete as needed.
5.4 Hepatic Toxicity
Elevations of ALT and AST (all grades) during the randomized treatment period occurred in 43% and 40%, respectively, with Grade 3 or 4 in 1% and 0%, respectively, of INREBIC-treated patients. The median time to onset of any grade transaminase elevation was approximately 1 month, with 75% of cases occurring within 3 months.
Monitor hepatic function at baseline, periodically during treatment, and as clinically indicated. For Grade 3 or higher ALT and/or AST elevations (greater than 5 × ULN), interrupt INREBIC dose until resolved to Grade 1 or less or to baseline. Restart dose at 100 mg daily below the last given dose. If re-occurrence of a Grade 3 or higher elevation of ALT/AST, discontinue treatment with INREBIC [see Dosage and Administration (2.5)].
5.5 Amylase and Lipase Elevation
Grade 3 or higher amylase and/or lipase elevations developed in 2% and 10%, respectively, of INREBIC-treated patients. The median time to onset of any grade amylase or lipase elevation was 15 days, with 75% of cases occurring within 1 month of starting treatment. One patient developed pancreatitis in the fedratinib clinical development program (n=608) and pancreatitis resolved with treatment discontinuation.
Monitor amylase and lipase at baseline, periodically during treatment, and as clinically indicated. For Grade 3 or higher amylase and/or lipase elevations, interrupt INREBIC until resolved to Grade 1 or less or to baseline. Restart dose at 100 mg daily below the last given dose [see Dosage and Administration (2.5)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Encephalopathy, including Wernicke's [see Warnings and Precautions (5.1)]
- Anemia and Thrombocytopenia [see Warnings and Precautions (5.2)]
- Gastrointestinal Toxicity [see Warnings and Precautions (5.3)]
- Hepatic Toxicity [see Warnings and Precautions (5.4)]
- Amylase and Lipase Elevation [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data in the WARNINGS AND PRECAUTIONS Section 5.1 Encephalopathy, including Wernicke's, reflect exposure to INREBIC as a single agent in 608 patients who received more than one dose (ranging from 30 mg to 800 mg) in Studies JAKARTA, ARD11936, JAKARTA2, ARD12042, ARD12888, TED12037/TED12015, INT12497, and TES13519, of whom 459 were patients with myelofibrosis, including 97 patients previously treated with ruxolitinib. Among the 608 patients receiving INREBIC, the median drug exposure was 37 weeks and the median number of cycles initiated was 9 cycles. Fifty-nine percent of 608 patients were exposed for 6 months or longer and 39% were exposed for 12 months or longer.
Using the dataset described above, the most common adverse reactions in >20% of patients (N=608) were diarrhea, nausea, anemia, vomiting, fatigue, thrombocytopenia, and constipation.
JAKARTA Trial
The safety of INREBIC was evaluated in the randomized treatment period of the JAKARTA trial [see Clinical Studies (14)]. Key eligibility criteria included adult patients with intermediate-2 or high-risk primary MF or post-PV MF or post-ET MF with splenomegaly, platelet count ≥50 × 109/L, and no splenectomy. Patients received INREBIC at 400 mg daily (n=96) or placebo (n=95). Among patients receiving INREBIC, 82% were exposed for more than 6 months and 65% for more than one year. Patients had a median duration of exposure to INREBIC 400 mg daily of 15.5 months compared with placebo where patients were treated for 6 months or until disease progression after which patients were allowed to crossover to active treatment. The median age of patients who received INREBIC was 65 years (range: 27 to 86 years), 59% were male, 90% were White, 8% were Asian, 1% were Black, 1% were Other, and 92% had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1.
Serious adverse reactions occurred in 21% of INREBIC-treated patients. Serious adverse reactions in ≥2% of patients receiving INREBIC 400 mg daily included cardiac failure (5%) and anemia (2%). Fatal adverse reactions of cardiogenic shock occurred in 1% of patients receiving INREBIC 400 mg daily.
Permanent discontinuation due to an adverse reaction occurred in 14% of patients receiving INREBIC. Most frequent reasons for permanent discontinuation in ≥2% of patients receiving INREBIC included cardiac failure (3%), thrombocytopenia, myocardial ischemia, diarrhea, and increased blood creatinine (2% each).
Dosage interruptions due to an adverse reaction during the randomized treatment period occurred in 21% of patients who received INREBIC. Adverse reactions requiring dosage interruption in >3% of patients who received INREBIC included diarrhea and nausea.
Dosage reductions due to an adverse reaction during the randomized treatment period occurred in 19% of patients who received INREBIC. Adverse reactions requiring dosage reduction in >2% of patients who received INREBIC included anemia (6%), diarrhea (3%), vomiting (3%), and thrombocytopenia (2%).
The most common adverse reactions (reported in ≥20%) were diarrhea, nausea, anemia, and vomiting.
Tables 3 and 4 summarize the common adverse reactions and laboratory abnormalities, respectively, in JAKARTA during randomized treatment.
Table 3: Adverse Reactions Reported in ≥5% Patients Receiving INREBIC 400 mg with a Difference between Arms of >5% during Randomized Treatment Adverse Reaction* INREBIC 400 mg
(n=96)Placebo
(n=95)All Grades
%Grade ≥3†
%All Grades
%Grade ≥3
%- * CTCAE version 4.03.
- † Only 1 Grade 4 event (anemia).
- ‡ Includes cystitis.
Diarrhea 66 5 16 0 Nausea 62 0 15 0 Anemia 40 30 14 7 Vomiting 39 3.1 5 0 Fatigue or asthenia 19 5 16 1.1 Muscle spasms 12 0 1.1 0 Blood creatinine increased 10 1 1.1 0 Pain in extremity 10 0 4.2 0 Alanine aminotransferase Increased 9 0 1.1 0 Headache 9 0 1.1 0 Weight increased 9 0 4.2 0 Dizziness 8 0 3.2 0 Bone pain 8 0 2.1 0 Urinary tract infection‡ 6 0 1.1 0 Dysuria 6 0 0 0 Aspartate aminotransferase increased 5 0 1.1 0 Clinically significant adverse reactions reported in 5% or less of patients: hypertension of all grades was reported in 4.2% of patients and Grade 3 or higher in 3% of INREBIC-treated patients.
Changes in selected post-baseline laboratory values that were observed are shown in Table 4 for the JAKARTA trial during randomized treatment.
Table 4: Selected Laboratory Abnormalities That Have Worsened from Baseline (≥20%) in Patients Receiving INREBIC with a Difference between Arms of >10% When Compared to Placebo in JAKARTA during Randomized Treatment INREBIC 400 mg
(n=96)Placebo
(n=95)Laboratory Parameter All Grades
%Grade ≥3
%All Grades
%Grade ≥3
%Hematology Anemia 74 34 32 10 Thrombocytopenia 47 12 26 10 Neutropenia 23 5 13 3.3 Biochemistry Creatinine increased 59 3.1 19 1.1 ALT increased 43 1 14 0 AST increased 40 0 16 1.1 Lipase increased 35 10 7 2.2 Hyponatremia 26 5 11 4.3 Amylase increased 24 2.1 5 0 -
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on INREBIC
Strong CYP3A4 Inhibitors
Coadministration of INREBIC with a strong CYP3A4 inhibitor increases fedratinib exposure [see Clinical Pharmacology (12.3)]. Increased exposure may increase the risk of adverse reactions [see Warnings and Precautions (5), and Adverse Reactions (6.1)]. Consider alternative therapies that do not strongly inhibit CYP3A4 activity. Alternatively, reduce the dose of INREBIC when administering with a strong CYP3A4 inhibitor [see Dosage and Administration (2.3)].
Strong and Moderate CYP3A4 Inducers
Avoid INREBIC with strong and moderate CYP3A4 inducers. The effect of concomitant administration of a strong or moderate CYP3A4 inducer with INREBIC has not been studied [see Clinical Pharmacology (12.3)].
Dual CYP3A4 and CYP2C19 Inhibitors
Avoid INREBIC with dual CYP3A4 and CYP2C19 inhibitor. The effect of concomitant administration of a dual CYP3A4 and CYP2C19 inhibitor with INREBIC has not been studied [see Clinical Pharmacology (12.3)].
7.2 Effect of INREBIC on Other Drugs
CYP3A4, CYP2C19, or CYP2D6 Substrate Drugs
Coadministration of INREBIC with drugs that are CYP3A4 substrates, CYP2C19 substrates, or CYP2D6 substrates increases the concentrations of these drugs, which may increase the risk of adverse reactions of these drugs [see Clinical Pharmacology (12.3)]. Monitor for adverse reactions and adjust the dose of drugs that are CYP3A4, CYP2C19, or CYP2D6 substrates as necessary when coadministered with INREBIC.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on INREBIC use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of fedratinib to pregnant rats during organogenesis at doses considerably lower than the recommended human daily dose of 400 mg/day resulted in adverse developmental outcomes (see Data). Consider the benefits and risks of INREBIC for the mother and possible risks to the fetus when prescribing INREBIC to a pregnant woman.
The background risk of major birth defects and miscarriage for the indicated population is unknown. Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In an embryo-fetal development study in pregnant rats, fedratinib administration at a dose of 30 mg/kg/day during organogenesis (gestation days 6 to 17) was associated with adverse developmental outcomes including skeletal variations (such as additional ossification center of neuronal arches). These effects occurred in rats at approximately 0.1 times the clinical exposure based on AUC at the recommended daily dose. At lower doses of 10 mg/kg/day (0.01 times the clinical exposure at the recommended daily dose), fedratinib administered to pregnant rats resulted in maternal toxicity of decreased gestational weight gain.
In an embryo-fetal development study in pregnant rabbits, fedratinib administration during organogenesis (gestation Days 6 to 18) did not produce developmental or maternal toxicity at doses up to the highest dose level tested, 30 mg/kg/day (approximately 0.08 times the clinical exposure at the recommended daily dose). In a separate study, administration of 80 mg/kg/day fedratinib to rabbits resulted in maternal mortality.
In a pre- and postnatal study in rats, fedratinib was administered to pregnant female rats at doses of 3, 10, or 30 mg/kg/day from Day 6 of gestation through Day 20 of lactation, with weaning on Day 21. A slight decrease in maternal body weight gain during gestation occurred at 30 mg/kg/day. The offspring from the high dose (30 mg/kg) had decreased body weight preweaning in both sexes and postweaning through the maturation phase in males. These effects occurred at exposures approximately 0.1 times the clinical exposure at the recommended daily dose.
8.2 Lactation
Risk Summary
There are no data on the presence of fedratinib or its metabolites in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise patients not to breastfeed during treatment with INREBIC, and for at least 1 month after the last dose.
8.4 Pediatric Use
The safety and effectiveness of INREBIC in pediatric patients have not been established.
8.5 Geriatric Use
Of the total number of patients with myelofibrosis who received an INREBIC dose of 400 mg in the clinical studies, 47.3% were greater than 65 years of age and 12.3% were greater than 75 years of age. No overall differences in safety or effectiveness of INREBIC were observed between these patients and younger patients.
8.6 Renal Impairment
Reduce INREBIC dose when administered to patients with severe renal impairment (CLcr 15 mL/min to 29 mL/min by Cockcroft-Gault) [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)]. No modification of the starting dose is recommended for patients with mild to moderate renal impairment (CLcr 30 mL/min to 89 mL/min by Cockcroft-Gault). Due to potential increase of exposure, patients with pre-existing moderate renal impairment require more intensive safety monitoring, and if necessary, dose modifications based on adverse reactions [see Dosage and Administration (2.5].
8.7 Hepatic Impairment
INREBIC pharmacokinetics has not been evaluated in patients with severe hepatic impairment (total bilirubin > 3 times ULN and any AST). Avoid use of INREBIC in patients with severe hepatic impairment [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
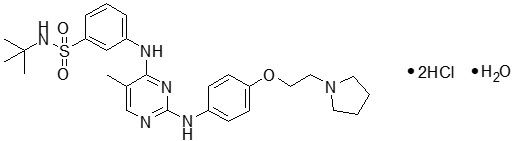
INREBIC (fedratinib) is a kinase inhibitor with the chemical name N-tert-butyl-3-[(5-methyl-2-{[4-(2-pyrrolidin-1-ylethoxy)phenyl]amino}pyrimidin-4-yl)amino]benzenesulfonamide dihydrochloride monohydrate. Its empirical formula is C27H36N6O3S∙2HCl∙H2O and a molecular weight of 615.62. Fedratinib exhibits pH-dependent aqueous solubility; it is freely soluble in the acidic condition (>100 mg/mL at pH 1) and practically insoluble in the neutral condition (4 mcg/mL at pH 7.4). The chemical structure is:
INREBIC (fedratinib) is available as 100-mg (equivalent to 117.3 mg of fedratinib dihydrochloride monohydrate) hard gelatin capsules for oral administration. Each capsule contains inactive ingredients of silicified microcrystalline cellulose and sodium stearyl fumarate. The capsule shell contains gelatin, red iron oxide, titanium dioxide and white ink.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fedratinib is an oral kinase inhibitor with activity against wild type and mutationally activated Janus Associated Kinase 2 (JAK2) and FMS-like tyrosine kinase 3 (FLT3). Fedratinib is a JAK2-selective inhibitor with higher inhibitory activity for JAK2 over family members JAK1, JAK3 and TYK2. Abnormal activation of JAK2 is associated with myeloproliferative neoplasms (MPNs), including myelofibrosis and polycythemia vera. In cell models expressing mutationally active JAK2V617F or FLT3ITD, fedratinib reduced phosphorylation of signal transducer and activator of transcription (STAT3/5) proteins, inhibited cell proliferation, and induced apoptotic cell death. In mouse models of JAK2V617F-driven myeloproliferative disease, fedratinib blocked phosphorylation of STAT3/5, and improved survival, white blood cell counts, hematocrit, splenomegaly, and fibrosis.
12.2 Pharmacodynamics
Fedratinib inhibited cytokine-induced STAT3 phosphorylation in whole blood from patients with myelofibrosis. The inhibition of STAT3 phosphorylation was maximal approximately 2 hours after the first dose, with values returning to near baseline at 24 hours. After daily administration of fedratinib, levels of inhibition at steady state PK were similar to the maximal inhibition reached after the first dose of 300 (0.75 times the recommended dose), 400 or 500 mg (1.25 times the recommended dose) of fedratinib.
12.3 Pharmacokinetics
INREBIC at 300 mg to 500 mg once daily (0.75 to 1.25 times the recommended dose) results in a dose proportional increase in geometric mean fedratinib peak concentrations (Cmax) and the area under the plasma concentration time curve over the dosing interval (AUCtau). The mean steady state levels are achieved within 15 days of daily dosing. The mean accumulation ratio ranged between 3- to 4-fold.
At the dose of 400 mg once daily, the geometric mean (coefficient of variation, %CV) fedratinib Cmax is 1804 ng/mL (49%) and AUCtau is 26870 ng.hr/mL (43%) in patients with myelofibrosis.
Absorption
Following 400 mg once daily, fedratinib median time to peak concentrations (Tmax) at steady-state is 3 hours (range: 2 to 4 hours).
Effect of Food
A low-fat, low-calorie (total 162 calories: 6% from fat, 78% from carbohydrate and 16% from protein) or a high-fat, high-calorie (total 815 calories: 52% from fat, 33% from carbohydrate and 15% from protein) meal increased area under the curve over time to infinity (AUCinf) up to 24% and Cmax up to 14% of a single 500 mg dose of fedratinib.
Distribution
The apparent volume of distribution of fedratinib at steady-state is 1770 L in patients with myelofibrosis at 400 mg once daily dose. Fedratinib is 92% or greater bound to human plasma proteins.
Elimination
Fedratinib pharmacokinetics is characterized by a biphasic disposition with an effective half-life of 41 hours, a terminal half-life of approximately 114 hours, and apparent clearance (CL/F) (%CV) of 13 L/hr (51%) in patients with myelofibrosis.
Specific Populations
Age (20 years to 95 years), race (White, Asians), sex, body weight (40 kg to 135 kg), mild [total bilirubin ≤upper limit of normal (ULN) and AST >ULN or total bilirubin 1 to 1.5 times ULN and any AST] or moderate (total bilirubin >1.5 to 3 times ULN and any AST) hepatic impairment, and mild (CLcr 60 mL/min to 89 mL/min by C-G) renal impairment did not have clinically meaningful effects on the pharmacokinetics of fedratinib.
The effect of severe (total bilirubin >3 times ULN and any AST) hepatic impairment on fedratinib pharmacokinetics is unknown.
Patients with Renal Impairment
Following a single 300 mg dose of INREBIC, the AUCinf of fedratinib increased by 1.5-fold in subjects with moderate (CLcr 30 mL/min to 59 mL/min by C-G) renal impairment and 1.9-fold in subjects with severe (CLcr 15 mL/min to 29 mL/min by C-G) renal impairment, compared to that in subjects with normal renal function (CLcr ≥90 mL/min by C-G) [see Dosage and Administration (2.4) and Renal Impairment (8.6)].
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Effect of Strong and Moderate CYP3A4 Inhibitors
Coadministration of ketoconazole (strong CYP3A4 inhibitor: 200 mg twice daily) with a single dose of INREBIC (300 mg) increased fedratinib AUCinf by 3-fold [see Dosage and Administration (2.3) and Drug Interactions (7.1)].
Based on modeling and simulation, coadministration of a strong CYP3A4 inhibitor such as ketoconazole (400 mg once daily) with INREBIC 400 mg once daily is predicted to increase fedratinib AUC at steady state by 2-fold [see Dosage and Administration (2.3) and Drug Interactions (7.1)].
Based on modeling and simulation, coadministration of moderate CYP3A4 inhibitors, erythromycin (500 mg three times daily) or diltiazem (120 mg twice daily), with INREBIC 400 mg once daily is predicted to increase fedratinib AUC at steady state by 1.2-, and 1.1-fold, respectively.
Effect of Dual CYP3A4 and CYP2C19 Inhibitor
The effect of concomitant administration with a dual CYP3A4 and CYP2C19 inhibitor on fedratinib pharmacokinetics is not known [see Drug Interactions (7.1)]
Effect of Strong and Moderate CYP3A4 Inducers
The effect of concomitant administration with a strong or moderate CYP3A4 inducer on fedratinib pharmacokinetics is not known [see Drug Interactions (7.1)].
Effect of Gastric Acid Reducing Agents
Coadministration of pantoprazole (proton pump inhibitor: 40 mg once daily) with a single dose of INREBIC (500 mg) increased fedratinib AUCinf by 1.2-fold.
Effect of Fedratinib on Drugs that are CYP3A, CYP2C19, or CYP2D6 Substrates
Coadministration of a single dose of midazolam (CYP3A substrate: 2 mg), omeprazole (CYP2C19 substrate: 20 mg), and metoprolol (CYP2D6 substrate: 100 mg) increased midazolam, omeprazole, or metoprolol AUCinf by 4-, 3-, and 2-fold, respectively [see Drug Interactions (7.2)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Fedratinib was not carcinogenic in the 6-month Tg.rasH2 transgenic mouse model.
Fedratinib was not mutagenic in a bacterial mutagenicity assay (Ames test) or clastogenic in in vitro chromosomal aberration assay (Chinese hamster ovary cells) or in vivo in a micronucleus test in rats.
In a fertility study in rats, fedratinib was administered for at least 70 days (males) and 14 days (females) prior to cohabitation and up to the implantation day (gestation day 7). Fedratinib had no effect on the estrous cycle parameters, mating performance, fertility, pregnancy rate or reproductive parameters in male or female rats at doses up to 30 mg/kg. The exposure (AUC) at the dose of 30 mg/kg/day is approximately 0.10 to 0.13 times the clinical exposure at the recommended daily dose.
-
14 CLINICAL STUDIES
JAKARTA
JAKARTA (NCT01437787) was a double-blind, randomized, placebo-controlled trial in patients with intermediate-2 or high-risk myelofibrosis, post-polycythemia vera myelofibrosis or post-essential thrombocythemia myelofibrosis with splenomegaly. A total of 289 patients were randomized to receive either INREBIC 500 mg (N=97), 400 mg (n=96) or placebo (n=96) once daily for at least 6 cycles. The median age was 65 years (range 27 to 86 years), 47% of patients were older than 65 years and 59% were male. Sixty-four percent (64%) of patients had primary MF, 26% had post-polycythemia vera MF, and 10% had post-essential thrombocythemia MF. Fifty-two percent (52%) of patients had intermediate-2 risk, and 48% had high-risk disease. The median baseline hemoglobin level was 10.2 g/dL. The median baseline platelet count was 214 × 109/L; 16% of patients had a platelet count <100 × 109/L and 84% of patients had a platelet count ≥100 × 109/L. Patients had a baseline median palpable spleen length of 15 cm. Patients had a baseline median spleen volume as measured by magnetic resonance imaging (MRI) or computed tomography (CT) of 2568 mL (range of 316 to 8244 mL) (the upper limit of normal is approximately 300 mL). Patients underwent MRI or CT spleen volume assessment (after the third and sixth cycle) with a follow-up scan 4 weeks after Cycle 6.
The efficacy of INREBIC in the treatment of patients with primary or secondary myelofibrosis was established based upon the proportion of patients achieving greater than or equal to a 35% reduction from baseline in spleen volume at the End of Cycle 6 as measured by MRI or CT with a follow-up scan 4 weeks later.
Efficacy analyses are presented in Table 5.
Table 5: Percent of Patients Achieving 35% or Greater Reduction from Baseline in Spleen Volume at the End of Cycle 6 in the Phase 3 Study, JAKARTA (ITT Population) Spleen Response by MRI/CT at the End of Cycle 6 with a Follow-up Scan 4 Weeks Later INREBIC 400 mg
N=96
n (%)Placebo
N=96
n (%)Number (%) of Patients with Spleen Volume Reduction by 35% or More 35 (37) 1 (1) p-value p<0.0001 Figure 1 shows the percent change in spleen volume from baseline for patients who have an evaluable MRI/CT at the End of Cycle 6.
N*: Subjects with available percent change in spleen volume at EOC6. Figure 1: Percent Change in Spleen Volume from Baseline at the End of Cycle 6 for Each Patient in the Phase 3 Study, JAKARTA 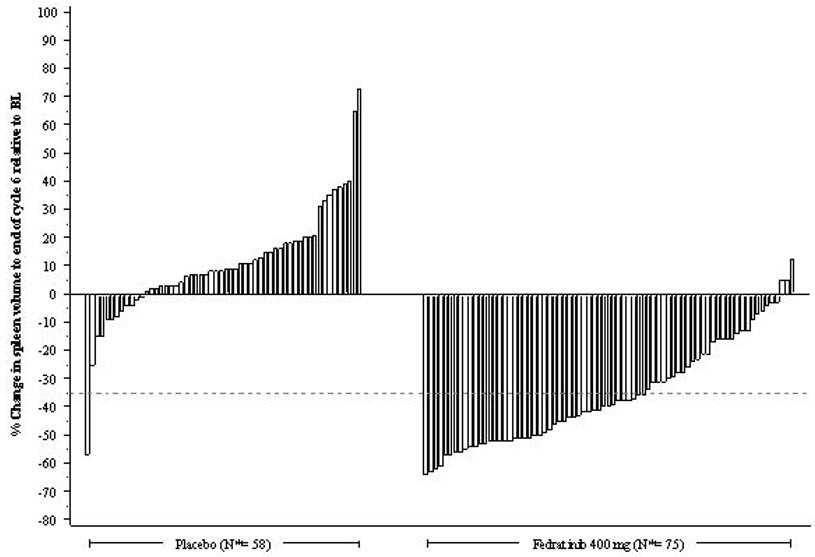
Based on Kaplan-Meier estimates, the median duration of spleen response was 18.2 months for the INREBIC 400 mg group.
Additional outcomes included the proportion of patients with a 50% or greater reduction in Total Symptom Score from baseline to the End of Cycle 6 as measured by the modified Myelofibrosis Symptom Assessment Form (MFSAF) v2.0 diary.
The modified MFSAF v2.0 is a patient diary capturing the 6 core symptoms of MF: night sweats, itching, abdominal discomfort, early satiety, pain under ribs on left side, and bone or muscle pain. The modified MFSAF diary was completed daily during the week prior to Day 1 of each treatment cycle, and at the End of Cycle 6. Symptom scores ranged from 0 ("absent") to 10 ("worst imaginable"). These scores were added to create the Total Symptom Score, which has a maximum score of 60. At baseline, the mean Total Symptom Score was 17.95 in the 400 mg group and 15.45 in the placebo group.
The proportion of patients with a 50% or greater reduction in Total Symptom Score was 40% in the INREBIC 400 mg group and 9% in the placebo group (Table 6). Results are excluded for 22 patients: 6 patients with a baseline Total Symptom Score of zero (2 in the INREBIC 400 mg group and 4 in the placebo group) and 16 patients with missing baseline (5 in the INREBIC 400 mg group and 11 in the placebo group).
Table 6: Improvement in Total Symptom Score in Patients with Myelofibrosis in the Phase 3 Study, JAKARTA INREBIC 400 mg
(N=89)
n (%)Placebo
(N=81)
n (%)Number (%) of Patients with 50% or Greater Reduction in Total Symptom Score at the End of Cycle 6 36 (40) 7 (9) p-value p<0.0001 Figure 2 shows the percent change in Total Symptom Score from baseline at the End of Cycle 6 for each patient.
N*: Subjects with available percent change in Total Symptom Score at EOC6. Figure 2: Percent Change from Baseline in Total Symptom Score at the End of Cycle 6 for Each Patient in the Phase 3 Study, JAKARTA 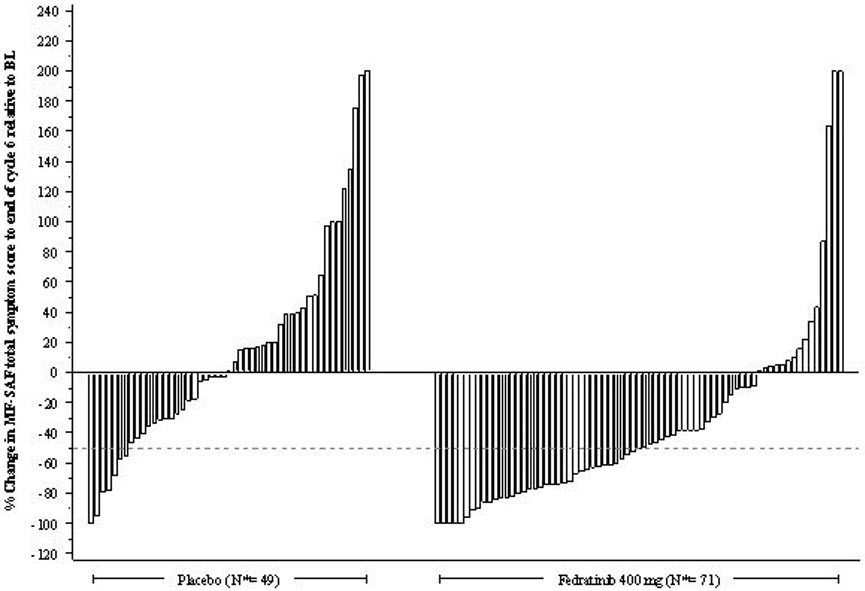
Figure 3 displays the proportion of patients with at least a 50% improvement in each of the individual symptoms that comprised the Total Symptom Score indicating that all 6 of the symptoms contributed to the higher Total Symptom Score response rate in the group treated with INREBIC.
Figure 3: Proportion of Patients Achieving 50% or Greater Reduction in Individual Symptom Scores at the End of Cycle 6 with Non-Zero Baseline Scores 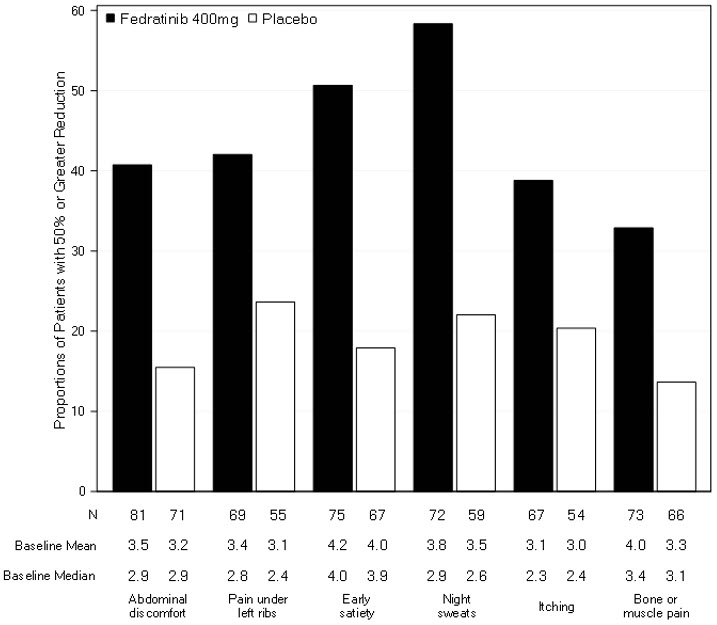
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
INREBIC (fedratinib) 100 mg capsules: Reddish brown, opaque, size 0 capsule, printed with "FEDR 100 mg" in white ink.
- Bottles of 120 capsules (NDC: 59572-720-12)
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Discuss the following with patients prior to and during treatment with INREBIC.
Encephalopathy, including Wernicke's
Advise patients that serious and fatal encephalopathy, including Wernicke's, has occurred in patients taking INREBIC. Wernicke's encephalopathy is a neurological emergency resulting from acute thiamine (Vitamin B1) deficiency. Advise patients of the need to monitor thiamine levels [see Dosage and Administration (2.1, 2.2, 2.6), and Warnings and Precautions (5.1)]. Advise patients to seek emergency medical attention for any change in mental status such as confusion, drowsiness or memory impairment, cerebellar abnormalities such as ataxia, and ophthalmic abnormalities such as diplopia and nystagmus. Advise patients to contact their healthcare provider right away if they experience nausea, vomiting, diarrhea, and weight loss unresponsive to treatment resulting in malnutrition and lower thiamine levels, which may lead to Wernicke's encephalopathy [see Boxed Warning and Warnings and Precautions (5.1)].
Anemia and Thrombocytopenia
Advise patients that INREBIC is associated with anemia and thrombocytopenia, and of the need to monitor complete blood counts before and during treatment [see Warnings and Precautions (5.2)].
Gastrointestinal Toxicity
Advise patients to contact their healthcare provider if they experience intractable diarrhea, nausea, or vomiting. Prescribers should advise patients of the potential complications of severe diarrhea, nausea, or vomiting [see Warnings and Precautions (5.3)].
Hepatic Toxicity
Advise patients that INREBIC may increase liver enzymes and of the need to monitor liver enzyme levels [see Warnings and Precautions (5.4)].
Amylase and Lipase Elevation
Advise patients that INREBIC may increase amylase and lipase and of the need to monitor amylase and lipase [see Warnings and Precautions (5.5)].
Lactation
Advise patients not to breastfeed during treatment with INREBIC and for at least 1 month after the final dose [see Use in Specific Populations (8.2)].
Dosing and Storage Instructions
- Instruct patients that if they miss a dose of INREBIC, skip the dose and take it the next day and return to normal schedule [see Dosage and Administration (2.1)]. Warn patients not to take 2 doses to make up for the missed dose.
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: Aug 2019 MEDICATION GUIDE
INREBIC® (inn-REH-bik)
(fedratinib)
capsules, for oral useWhat is the most important information I should know about INREBIC?
INREBIC may cause serious side effects, including:-
Encephalopathy (including Wernicke's encephalopathy). A serious and sometimes fatal neurological problem called encephalopathy (including Wernicke's encephalopathy) has happened in some people who take INREBIC.
Wernicke's encephalopathy is a neurologic emergency that can happen if you do not have enough vitamin B1 (thiamine) in your body. Your healthcare provider will do a blood test to check your vitamin B1 level before starting and during treatment with INREBIC. Your healthcare provider may tell you to stop taking INREBIC and take a vitamin B1 supplement if you develop side effects during treatment with INREBIC.
Call your healthcare provider right away if you develop diarrhea, nausea, or vomiting that does not respond to treatment.
Get emergency medical help right away if you develop the following:- confusion, memory problems or drowsiness
- problems with balance and movement, such as difficulty walking
- eye problems, such as double or blurred vision or abnormal eye movements
What is INREBIC?
INREBIC is a prescription medicine used to treat adults with certain types of myelofibrosis (MF).
It is not known if INREBIC is safe and effective in children.Before taking INREBIC, tell your healthcare provider about all your medical conditions, including if you: - have low red blood cell or platelet counts
- have or have had liver problems
- have or have had kidney problems
- are breastfeeding or plan to breastfeed. It is not known if INREBIC passes into your breast milk. You should not breastfeed during treatment with INREBIC and for at least 1 month after your last dose. Talk to your healthcare provider about the best way to feed your baby during treatment with INREBIC.
INREBIC and other medicines may affect each other causing unwanted side effects. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.How should I take INREBIC? - Take INREBIC exactly as your healthcare provider tells you to. Do not change your dose or stop taking INREBIC unless your healthcare provider tells you to.
- Take INREBIC 1 time each day.
- Take INREBIC with or without food. Taking INREBIC with a high fat meal may help to reduce nausea and vomiting symptoms.
- If you miss a dose of INREBIC, skip the missed dose and take your next dose at your regular time. Do not take 2 doses to make up for the missed dose.
What are the possible side effects of INREBIC?
INREBIC can cause serious side effects, including:- See "What is the most important information I should know about INREBIC?"
- Low blood cell counts. INREBIC may cause low red blood cell counts (anemia) and low platelet counts (thrombocytopenia) in some people. You may need a blood transfusion if your blood counts drop too low. Your healthcare provider will do blood tests to check your blood counts before you start and during treatment with INREBIC. Tell your healthcare provider if you develop any bleeding or bruising during treatment with INREBIC.
- Nausea, vomiting, and diarrhea. Your healthcare provider may give you certain medicines to help treat your nausea, vomiting, and diarrhea. Call your healthcare provider or get emergency medical help right away if you have nausea, vomiting, or diarrhea that does not get better with treatment.
- Liver problems. Your healthcare provider will do blood tests to check your liver function before starting and during treatment with INREBIC.
- Amylase and lipase increases. You may have changes in your blood amylase or lipase levels that may indicate a problem with your pancreas. Your healthcare provider will do blood tests to check your amylase or lipase levels before starting and during treatment with INREBIC.
- diarrhea
- nausea
- low red blood cell counts (anemia)
- vomiting
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store INREBIC? - Store INREBIC below 86°F (30°C).
General information about the safe and effective use of INREBIC.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use INREBIC for conditions for which it was not prescribed. Do not give INREBIC to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about INREBIC that is written for health professionals.What are the ingredients in INREBIC?
Active ingredient: fedratinib
Inactive ingredients: silicified microcrystalline cellulose and sodium stearyl fumarate. The capsule shell contains gelatin, red iron oxide, titanium dioxide and white ink.
Manufactured for and marketed by:
Celgene Corporation, Summit, NJ 07901
INREBIC® is a registered trademark of Impact Biomedicines, Inc., a wholly owned subsidiary of Celgene Corporation
Pat. www.celgene.com/therapies © 2018 - 2019 Impact Biomedicines, Inc. All Rights Reserved. INRMG.001 08/2019
For more information go to www.INREBIC.com or call 1-888-423-5436. -
Encephalopathy (including Wernicke's encephalopathy). A serious and sometimes fatal neurological problem called encephalopathy (including Wernicke's encephalopathy) has happened in some people who take INREBIC.
-
PRINCIPAL DISPLAY PANEL - 100 mg Capsule Bottle Label
NDC: 59572-720-12
INREBIC®
(fedratinib) capsules100 mg
Dispense the accompanying Medication Guide to each patient.
Rx only
120 Capsules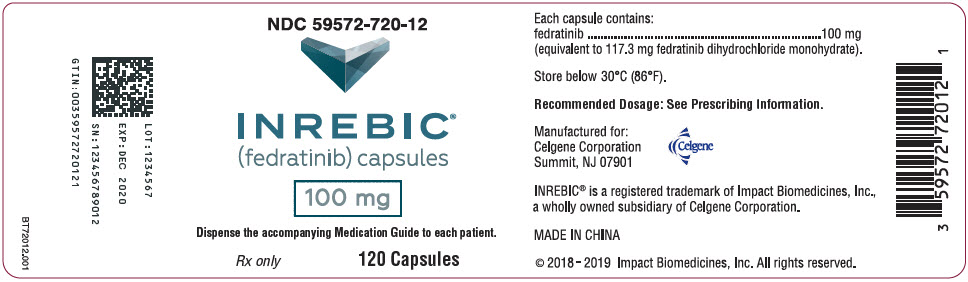
-
INGREDIENTS AND APPEARANCE
INREBIC
fedratinib hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59572-720 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEDRATINIB HYDROCHLORIDE (UNII: UH9J2HBQWJ) (FEDRATINIB - UNII:6L1XP550I6) FEDRATINIB 100 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BROWN (Reddish brown) Score no score Shape CAPSULE (Shape Description) Size 22mm Flavor Imprint Code FEDR;100;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59572-720-12 120 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/16/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212327 08/16/2019 Labeler - Celgene Corporation (174201137)
Trademark Results [Inrebic]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 INREBIC 88207119 not registered Live/Pending |
Celgene Corporation 2018-11-27 |
 INREBIC 86690908 4931750 Live/Registered |
IMPACT BIOMEDICINES, INC. 2015-07-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.