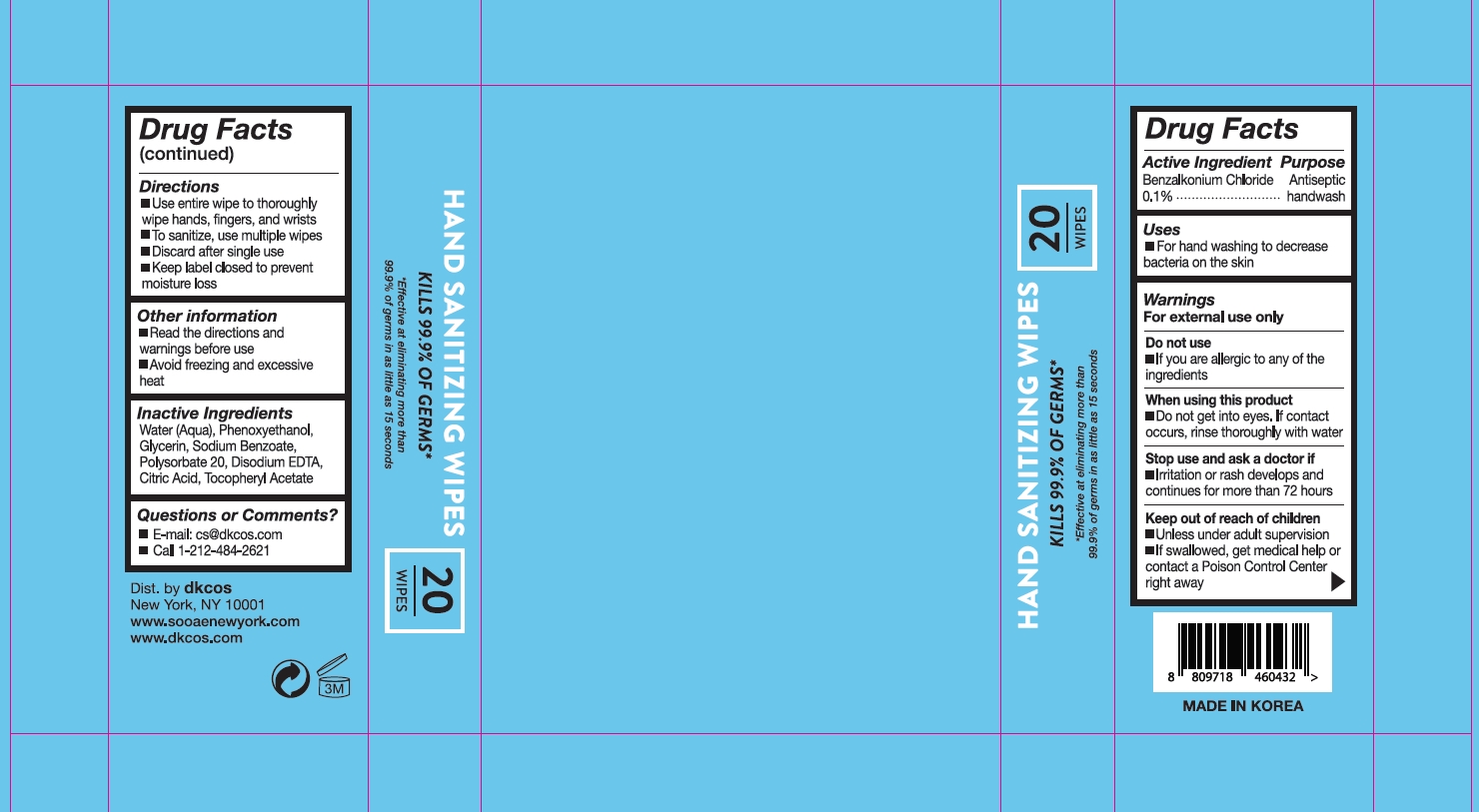
Soo'AE Hand Sanitizing Wipes 20
SooAE HAND SANTIZING WIPES by
Drug Labeling and Warnings
SooAE HAND SANTIZING WIPES by is a Otc medication manufactured, distributed, or labeled by DKCOS CORP. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SOOAE HAND SANTIZING WIPES- benzalkonium chloride clothÂ
DKCOS CORP
----------
Soo'AE Hand Sanitizing Wipes 20
Directions
- Use entire wipe to thoroughly wipe hands, fingers, and wrists
- To sanitize, use multiple wipes
- Discard after single use
- Keep label closed to prevent moisture loss
| SOOAE HAND SANTIZING WIPESÂ
benzalkonium chloride cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler -Â DKCOS CORP (114993964) |
Revised: 12/2024
Â
Document Id: 2a5e4474-e805-a08d-e063-6294a90a0fec
Set id: f0fe988b-e2e0-df4b-e053-2995a90a115a
Version: 3
Effective Time: 20241231
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.