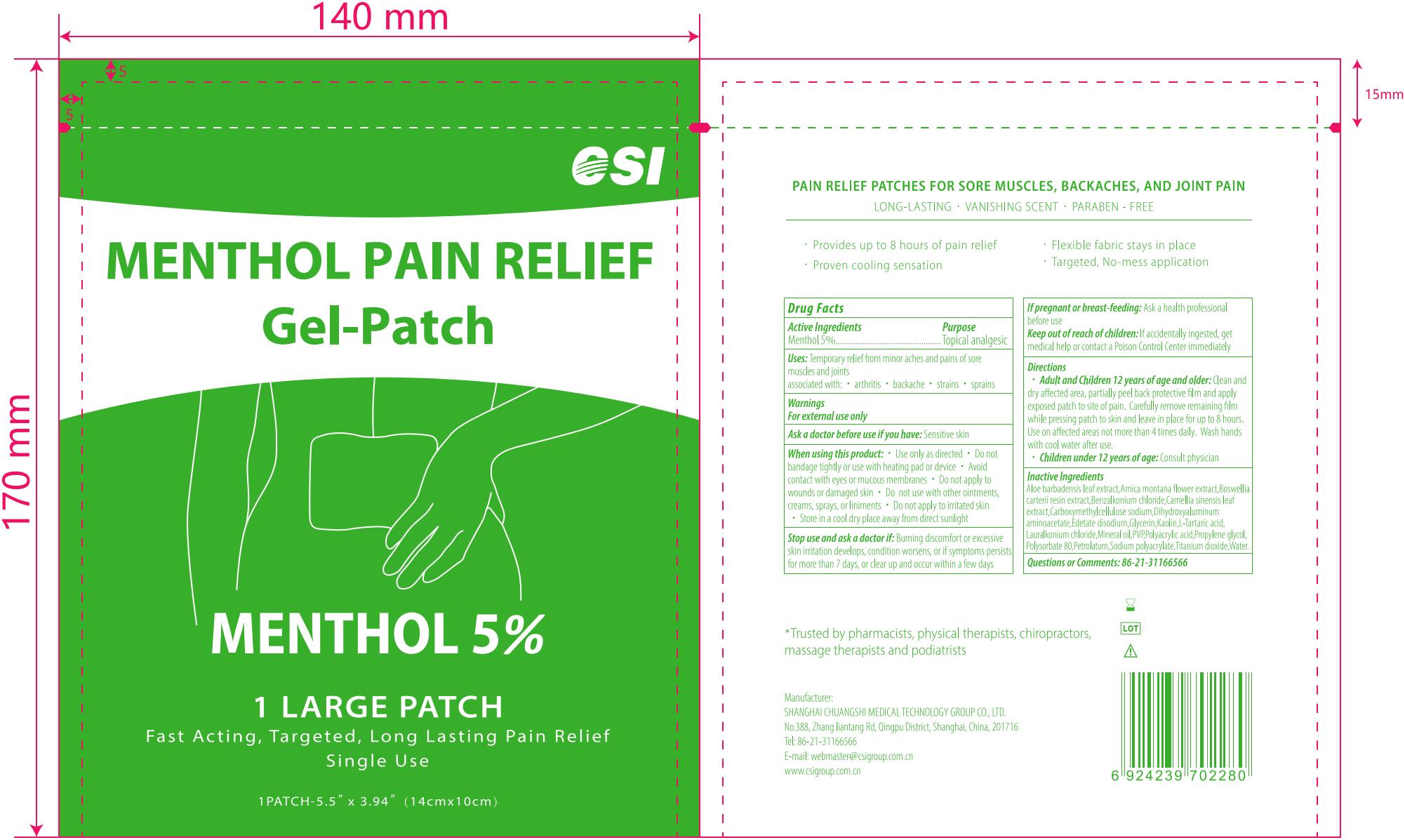
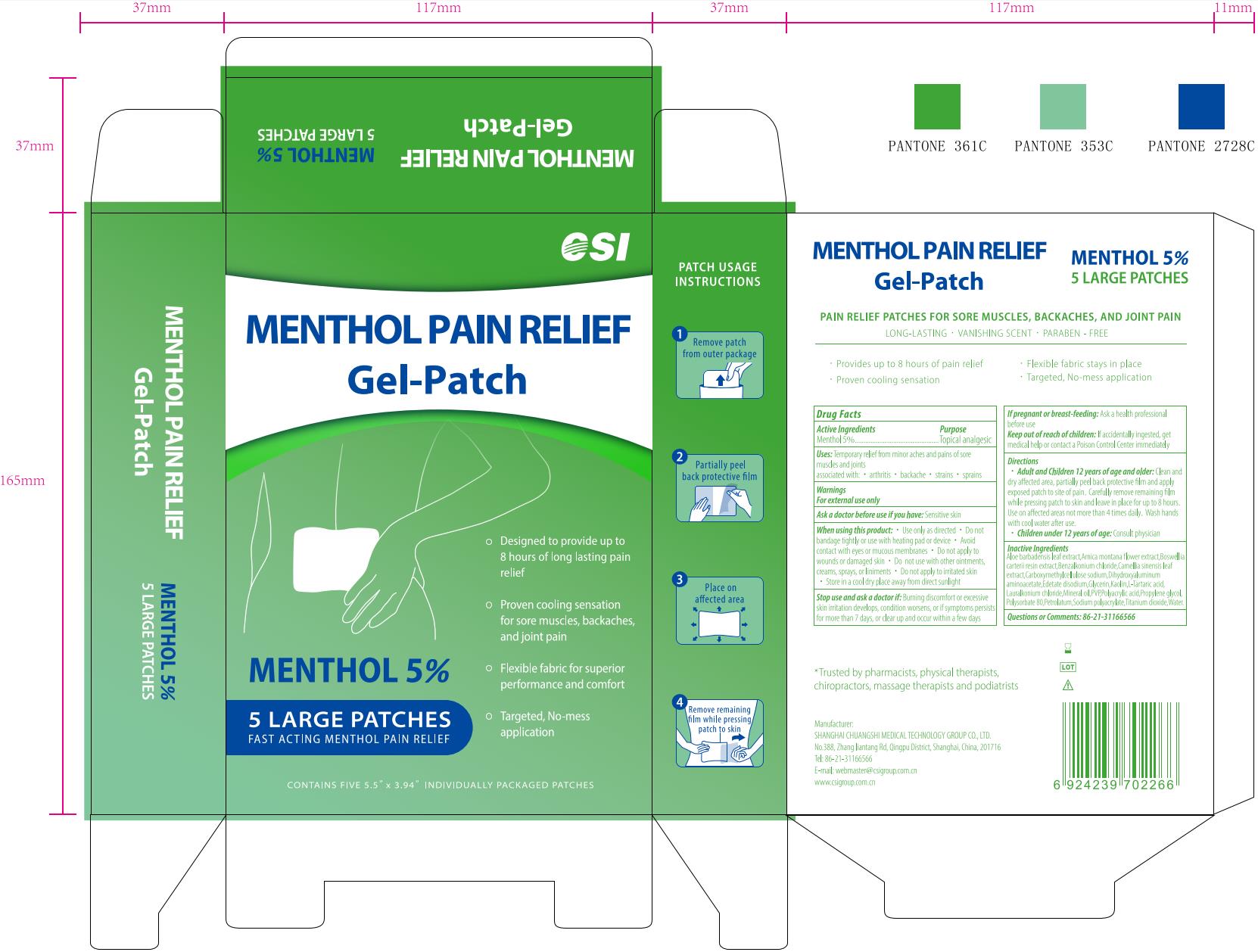
CSI MENTHOL PAIN RELIEF Gel-Patch, 5 Patches
MENTHOL PAIN RELIEF Gel-Patch by
Drug Labeling and Warnings
MENTHOL PAIN RELIEF Gel-Patch by is a Otc medication manufactured, distributed, or labeled by Shanghai Chuangshi Medical Technology (Group) Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MENTHOL PAIN RELIEF GEL-PATCH- menthol patch
Shanghai Chuangshi Medical Technology (Group) Co., Ltd.
----------
CSI MENTHOL PAIN RELIEF Gel-Patch, 5 Patches
Uses
Temporary relief from minor aches and pains of sore muscles and joints associated with:
- arthrits
- backache
- strains
- sprains
Warnings
- For external use only
- Use only as directed
- Do not bandage tightly or use with heating pad or device
- Avoid contact with eyes or mucous membranes
- Do not apply to wounds or damaged skin
- Do not use with other ointments, creams, sprays, or liniments
- Do not apply to irritated skin
Ask doctor
Ask a doctor before use if you have: Sensitive skin
Stop use and ask a doctor if: Burning disconfort or excessive skin irritation develops, condition worsens, or if symptoms persist for more than 7 days, or clear up and occur again within a few days
If pregnant or breast-feeding: Ask a health professional before use
When using
For external use only
- Use only as directed
- Do not bandage tightly or use with heating pad or device
- Avoid contact with eyes or mucous membranes
- Do not apply to wounds or damaged skin
- Do not use with other ointments, creams, sprays, or liniments
- Do not apply to irritated skin
Do not use
- Use only as directed
- Do not bandage tightly or use with heating pad or device
- Avoid contact with eyes or mucous membranes
- Do not apply to wounds or damaged skin
- Do not use with other ointments, creams, sprays, or liniments
- Do not apply to irritated skin
Stop use
Stop use and ask a doctor if: Burning disconfort or excessive skin irritation develops, condition worsens, or if symptoms persist for more than 7 days, or clear up and occur again within a few days
Keep out of reach of children
Keep out of reach of children: If accidentally ingested, get medical help or contact a Poison Control Center immediately
Directions
- Adult and Children 12 years of age and older: Clean and dry affected area, partially peel back protective film and apply exposed patch to site of pain. Carefully remove remaining film while pressing patch to skin and leave in place for up to 8 hours. Use on affected areas not more than 4 times daily. Wash hands with cool water after use.
- Children under 12 years of age: Consult physician
Inactive ingredients
Water, Glycerin, Polyacrylic acid, Propylene glycol, Sodium polyacrylate, Mineral oil, Polysorbate 80, PVP, Petrolatum, Dihydroxyaluminum aminoacetate, Edetate disodium, Kaolin, Carboxymethylcellulose sodium, Titanium dioxide,
L-Tartaric acid, Benzalkonium chloride, Lauralkonium chloride, Aloe barbadensis leaf extract, Arnica montana flower extract, Boswellia carterii resin extract, Camellia sinensis leaf extract
| MENTHOL PAIN RELIEF GEL-PATCH
menthol patch |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Shanghai Chuangshi Medical Technology (Group) Co., Ltd. (546872672) |
| Registrant - Shanghai Chuangshi Medical Technology (Group) Co., Ltd. (546872672) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shanghai Chuangshi Medical Technology (Group) Co., Ltd. | 546872672 | manufacture(73557-132) , label(73557-132) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.