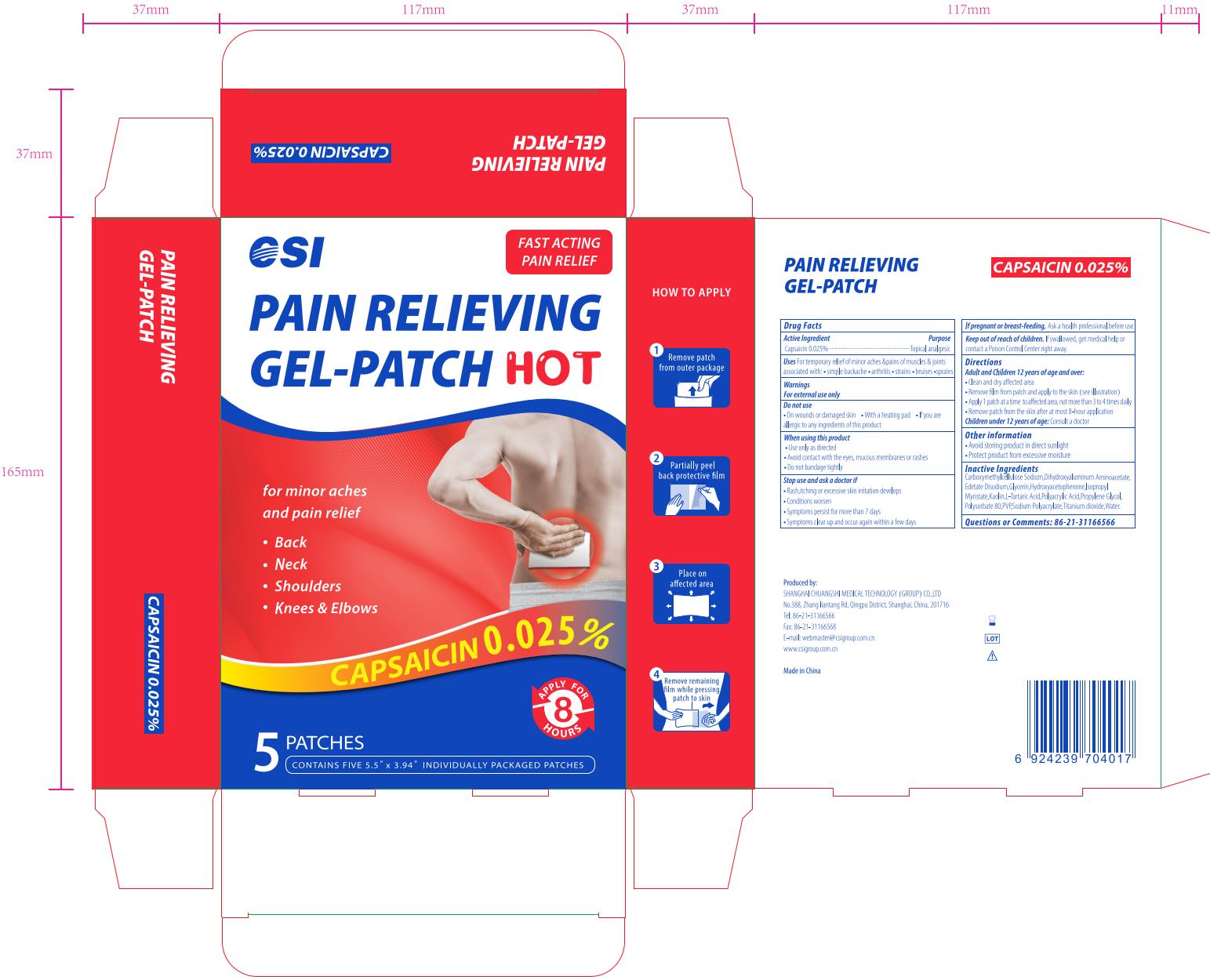
CSI Nonivamide Pain Relieving Gel-Patch, 5 Patches
Pain Relieving Gel-Patch by
Drug Labeling and Warnings
Pain Relieving Gel-Patch by is a Otc medication manufactured, distributed, or labeled by Shanghai Chuangshi Medical Technology (Group) Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PAIN RELIEVING GEL-PATCH- nonivamide patch
Shanghai Chuangshi Medical Technology (Group) Co., Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
CSI Nonivamide Pain Relieving Gel-Patch, 5 Patches
Uses
For temporary relief of minor aches & pains of muscles & joints associated with:
- simple backache
- arthritis
- stratins
- bruises
- sprains
Do not use
- On wounds or damaged skin
- With a heating pad
- If you are allergic to any ingredients of this product
When using this product
- Use only as directed
- Avoid contact with the eyes, mucous membranes or rashes
- Do not bandage tightly
Stop use and ask a doctor if
- Rash, itching or excessive skin irratation develops
- Condition worsen
- Symptoms persist for more than 7 days
- Symptoms clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away
Directions
Adult and Children 12 years of age and over:
- Clean and dry affected area
- Remove film from patch and apply to the skin (see illustration)
- Apply 1 patch at a time to affected area, not more than 3 to 4 times daily
- Remove patch from the skin after at most 8-hour application
Children under 12 years of age: Consult a doctor
| PAIN RELIEVING GEL-PATCH
nonivamide patch |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Shanghai Chuangshi Medical Technology (Group) Co., Ltd. (546872672) |
| Registrant - Shanghai Chuangshi Medical Technology (Group) Co., Ltd. (546872672) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shanghai Chuangshi Medical Technology (Group) Co., Ltd. | 546872672 | manufacture(73557-183) , label(73557-183) | |
Revised: 11/2023
Document Id: 0a15627b-60c1-de5a-e063-6394a90abd4c
Set id: f104e750-b475-657e-e053-2995a90a2855
Version: 4
Effective Time: 20231113
Shanghai Chuang
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.