FOSRENOL- lanthanum carbonate tablet, chewable FOSRENOL- lanthanum carbonate powder
Fosrenol by
Drug Labeling and Warnings
Fosrenol by is a Prescription medication manufactured, distributed, or labeled by Takeda Pharmaceuticals America, Inc., Patheon Manufacturing Services LLC, Element Materials Technology Pharma US LLC, Reading Scientific Services Ltd (RSSL), Triclinic Labs, Microbac Laboratories, Inc., Catalent Germany Schorndorf GmbH, BCM Ltd, Reckitt Benckiser Healthcare International Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FOSRENOL safely and effectively. See full prescribing information for FOSRENOL.
FOSRENOL (lanthanum carbonate) chewable tablets, for oral use
FOSRENOL (lanthanum carbonate) oral powder, for oral use
Initial U.S. Approval: 2004RECENT MAJOR CHANGES
Warnings and Precautions (5.1) 11/2018 INDICATIONS AND USAGE
- FOSRENOL is a phosphate binder indicated to reduce serum phosphate in patients with end stage renal disease (ESRD). (1)
DOSAGE AND ADMINISTRATION
- The recommended initial total daily dose of FOSRENOL is 1500 mg in divided doses. Titrate every 2-3 weeks based on serum phosphate level. (2)
- Take FOSRENOL with or immediately after meals. (2)
- FOSRENOL Chewable Tablets: Chew or crush tablet completely before swallowing. (2)
- FOSRENOL Oral Powder: Sprinkle powder on a small quantity of applesauce or other similar food and consume immediately. Consider powder formulation in patients with poor dentition or who have difficulty chewing tablets (2)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- Bowel obstruction, ileus, and fecal impaction. (4)
WARNINGS AND PRECAUTIONS
- Serious cases of gastrointestinal obstruction, ileus, subileus, gastrointestinal perforation, and fecal impaction. Risks include altered gastrointestinal anatomy, hypomotility disorders, and concomitant medications. Advise patients to chew or crush the tablet completely. (5.1)
- FOSRENOL has radio-opaque properties and therefore may give the appearance typical of an imaging agent during abdominal X-ray procedures. (5.2)
ADVERSE REACTIONS
- In controlled trials, the most common adverse reactions that were more frequent (≥ 5% difference vs. placebo) in FOSRENOL were nausea, vomiting and abdominal pain. (6.1)
- The following adverse reactions have been identified during post-approval use of FOSRENOL: constipation, dyspepsia, allergic skin reactions, and tooth injury while chewing the tablet. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Shire US Inc. at 1-800-828-2088 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- There is a potential for FOSRENOL to interact with compounds that bind to cationic antacids (i.e., aluminum-, magnesium-, or calcium-based). Therefore, do not take such compounds within 2 hours of dosing with FOSRENOL. (7.1)
- Oral quinolone antibiotics must be taken at least 1 hour before or 4 hours after FOSRENOL. (7.2)
- Do not take thyroid hormone replacement therapy within 2 hours of dosing with FOSRENOL. Monitoring of TSH levels is recommended in patients receiving both medicinal agents. (7.3)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Gastrointestinal Adverse Effects
5.2 Diagnostic Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs Binding to Antacids
7.2 Quinolone Antibiotics
7.3 Levothyroxine
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.3 Developmental Toxicity
14 CLINICAL STUDIES
14.1 Double-Blind Placebo-Controlled Studies
14.2 Open-Label Active-Controlled Studies
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 FOSRENOL Chewable Tablets
16.2 FOSRENOL Oral Powder
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
FOSRENOL is a phosphate binder indicated to reduce serum phosphate in patients with end stage renal disease (ESRD).
Management of elevated serum phosphorus levels in end stage renal disease patients usually includes all of the following: reduction in dietary intake of phosphate, removal of phosphate by dialysis and reduction of intestinal phosphate absorption with phosphate binders.
-
2 DOSAGE AND ADMINISTRATION
Divide the total daily dose of FOSRENOL and take with or immediately after meals. The recommended initial total daily dose of FOSRENOL is 1500 mg. Titrate the dose every 2-3 weeks until an acceptable serum phosphate level is reached. Monitor serum phosphate levels as needed during dose titration and on a regular basis thereafter.
In clinical studies of ESRD patients, FOSRENOL doses up to 4500 mg were evaluated. Most patients required a total daily dose between 1500 mg and 3000 mg to reduce plasma phosphate levels to less than 6.0 mg/dL. Doses were generally titrated in increments of 750 mg/day.
Information for FOSRENOL Chewable Tablets
Chew or crush FOSRENOL Chewable Tablets completely before swallowing. Do not swallow intact FOSRENOL Chewable Tablets.
Information for FOSRENOL Oral Powder
Sprinkle FOSRENOL Oral Powder on a small quantity of applesauce or other similar food and consume immediately. Do not open until ready to use. Do not store FOSRENOL Oral Powder for future use once mixed with food. As FOSRENOL Oral Powder is insoluble, do not attempt to dissolve in liquid for administration.
Consider using the oral powder formulation in patients with poor dentition or who have difficulty chewing tablets.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Gastrointestinal Adverse Effects
Serious cases of gastrointestinal obstruction, ileus, subileus, gastrointestinal perforation and fecal impaction have been reported in patients taking lanthanum, some requiring surgery or hospitalization. Consider discontinuing FOSRENOL in patients without another explanation for severe gastrointestinal symptoms.
Risk factors for gastrointestinal obstruction and gastrointestinal perforation identified from post-marketing reports in patients taking FOSRENOL Chewable Tablets include altered gastrointestinal anatomy (e.g., diverticular disease, peritonitis, history of gastrointestinal surgery, gastrointestinal cancer, gastrointestinal ulceration), hypomotility disorders (e.g., constipation, ileus, subileus, diabetic gastroparesis), and the use of medications known to potentiate these effects. Some cases were reported in patients with no history of gastrointestinal disease.
Patients with acute peptic ulcer, ulcerative colitis, Crohn's disease or bowel obstruction were not included in FOSRENOL clinical studies [see Contraindications (4)].
Advise patients who are prescribed FOSRENOL Chewable Tablets to chew the tablet completely and not to swallow them whole. Serious gastrointestinal complications have been reported in association with unchewed or incompletely chewed tablets [see Adverse Reactions (6.2)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Gastrointestinal Adverse Effects [see Warnings and Precautions (5.1)]
Overall, the safety profile of FOSRENOL has been studied in over 5200 subjects in completed clinical trials. The most common adverse reactions for FOSRENOL were gastrointestinal events, such as nausea, vomiting, and abdominal pain and they generally abated over time with continued dosing.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In double-blind, placebo-controlled studies where a total of 180 and 95 ESRD patients were randomized to FOSRENOL chewable tablet and placebo, respectively, for 4-6 weeks of treatment, the most common reactions that were more frequent (≥5% difference) in the FOSRENOL group were nausea, vomiting, and abdominal pain (Table 1).
Table 1. Adverse Reactions* That Were More Common on FOSRENOL in Placebo-Controlled, Double-Blind Studies With Treatment Periods of 4-6 Weeks FOSRENOL
%
(N=180)Placebo
%
(N=95)- * expressed as the event rate for each term
Nausea 11 5 Vomiting 9 4 Abdominal pain 5 0 In an open-label long-term 2 year extension study in 93 patients who had transitioned from other studies, resulting in a total of up to 6 years treatment, mean baseline values and changes in transaminases were similar to those observed in the earlier comparative studies, with little change during treatment.
The safety of FOSRENOL was studied in two long-term, open-labeled clinical trials, which included 1215 patients treated with FOSRENOL and 944 with alternative therapy. Fourteen percent (14%) of FOSRENOL treated patients discontinued treatment due to adverse events. Gastrointestinal adverse reactions, such as nausea, diarrhea and vomiting were the most common types of event leading to discontinuation.
In pooled active comparator controlled clinical trials, hypocalcemia was noted with an incidence of approximately 5% in both lanthanum and active comparator groups. A nonclinical study and a phase 1 study have shown reduced absorption of calcium in the intestine with lanthanum carbonate treatment.
In a crossover study in 72 healthy individuals comparing Fosrenol chewable tablets to Fosrenol oral powder, gastrointestinal adverse reactions such as nausea, diarrhea and vomiting were more common for the oral powder formulation (18%) than for the chewable tablets (7%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of FOSRENOL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: constipation, intestinal perforation, intestinal obstruction, ileus, subileus, dyspepsia, allergic skin reactions, hypophosphatemia, and tooth injury while chewing the tablet.
-
7 DRUG INTERACTIONS
Lanthanum in FOSRENOL has the potential to bind to drugs with anionic (e.g., carboxyl, carbonyl and hydroxyl) groups. FOSRENOL may decrease the bioavailability of tetracyclines or fluoroquinolones via this mechanism.
There are no empirical data on avoiding drug interactions between FOSRENOL and most concomitant oral drugs. For oral medications where a reduction in the bioavailability of that medication would have a clinically significant effect on its safety or efficacy, consider separation of the timing of the administration of the two drugs. The duration of separation depends upon the absorption characteristics of the medication concomitantly administered, such as the time to reach peak systemic levels and whether the drug is an immediate release or an extended release product. Consider monitoring clinical responses or blood levels of concomitant medications that have a narrow therapeutic range.
7.1 Drugs Binding to Antacids
There is a potential for FOSRENOL to interact with compounds which bind to cationic antacids (i.e., aluminum-, magnesium-, or calcium-based). Therefore, do not administer such compounds within 2 hours of dosing with FOSRENOL. Examples of relevant classes of compounds where antacids have been demonstrated to reduce bioavailability include antibiotics (such as quinolones, ampicillin and tetracyclines), thyroid hormones, ACE-inhibitors, statin lipid regulators and anti-malarials.
7.2 Quinolone Antibiotics
Co-administration of FOSRENOL with quinolone antibiotics may reduce the extent of their absorption. The bioavailability of oral ciprofloxacin was decreased by approximately 50% when taken with FOSRENOL in a single dose study in healthy volunteers. Administer oral quinolone antibiotics at least 1 hour before or 4 hours after FOSRENOL. When oral quinolones are given for short courses, consider eliminating the doses of FOSRENOL that would be normally scheduled near the time of quinolone intake to improve quinolone absorption [see Clinical Pharmacology (12.3)].
7.3 Levothyroxine
The bioavailability of levothyroxine was decreased by approximately 40% when taken together with FOSRENOL. Administer thyroid hormone replacement therapy at least 2 hours before or 2 hours after dosing with FOSRENOL and monitor thyroid stimulating hormone (TSH) levels [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. No adequate and well-controlled studies have been conducted in pregnant women. The effect of FOSRENOL on the absorption of vitamins and other nutrients has not been studied in pregnant women. FOSRENOL is not recommended for use during pregnancy.
Studies in pregnant rabbits showed that oral administration of lanthanum carbonate at 1500 mg/kg/day (5 times the maximum recommended daily human dose (MRHD) of 5725 mg, on a mg/m2 basis, assuming a 60 kg patient) was associated with increased post-implantation loss, reduced fetal weights, and delayed fetal ossification [see Nonclinical Toxicology (13.3)].
8.2 Labor and Delivery
No FOSRENOL treatment-related effects on labor and delivery were seen in animal studies. The effects of FOSRENOL on labor and delivery in humans is unknown.
8.3 Nursing Mothers
It is not known whether lanthanum carbonate is excreted in human milk. As many drugs are excreted in human milk, consider the possibility of infant exposure when FOSRENOL is administered to a nursing woman.
8.4 Pediatric Use
The safety and efficacy of FOSRENOL in pediatric patients have not been established. While growth abnormalities were not identified in long-term animal studies, lanthanum was deposited into developing bone including growth plate. The consequences of such deposition in developing bone in pediatric patients are unknown. Therefore, the use of FOSRENOL in this population is not recommended.
-
10 OVERDOSAGE
The symptoms associated with overdose are adverse reactions such as headache, nausea and vomiting. In clinical trials in healthy adults, GI symptoms were reported with daily doses up to 6000 mg/day of lanthanum carbonate administered with food. Given the topical activity of lanthanum in the gut, and the excretion in feces of the majority of the dose, supportive therapy is recommended for overdosage. Lanthanum carbonate was not acutely toxic in animals by the oral route. No deaths and no adverse effects occurred in mice, rats or dogs after single oral doses of 2000 mg/kg (1.7, 3.4, and 11.3 times the MRHD, respectively, on a mg/m2 basis).
-
11 DESCRIPTION
FOSRENOL contains lanthanum carbonate with molecular formula La2(CO3)3 xH2O (on average x=4-5 moles of water) and molecular weight 457.8 (anhydrous mass). Lanthanum carbonate is described as white to almost white powder. Lanthanum carbonate is practically insoluble in water and is insoluble in organic solvents; it dissolves in dilute mineral acids with effervescence.
Each FOSRENOL, white to off-white, chewable tablet contains lanthanum carbonate hydrate equivalent to 500, 750, or 1000 mg of elemental lanthanum and the following inactive ingredients: colloidal silicon dioxide NF, dextrates (hydrated) NF, magnesium stearate NF.
FOSRENOL Oral Powder is a white to off-white powder containing lanthanum carbonate hydrate equivalent to 750 or 1000 mg of elemental lanthanum and the following inactive ingredients: colloidal silicon dioxide NF, dextrates (hydrated) NF, magnesium stearate NF.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
FOSRENOL is a phosphate binder that reduces absorption of phosphate by forming insoluble lanthanum phosphate complexes that pass through the gastrointestinal (GI) tract unabsorbed. Both serum phosphate and calcium phosphate product are reduced as a consequence of the reduced dietary phosphate absorption.
12.2 Pharmacodynamics
In vitro studies have shown that lanthanum binds phosphate in the physiologically relevant pH range of 3 to 7. In simulated gastric fluid, lanthanum binds approximately 97% of the available phosphate at pH 3-5 and 67% at pH 7, when lanthanum is present in a two-fold molar excess to phosphate. Bile acids have not been shown to affect the phosphate binding affinity of lanthanum. In order to bind dietary phosphate, FOSRENOL must be administered with or immediately after meals.
In five Phase I pharmacodynamic studies comparing the reduction from baseline of urinary phosphorus excretion in healthy volunteers (N=143 taking lanthanum carbonate), it was shown that the mean intestinal phosphate binding capacity of lanthanum ranged from 235 to 468 mg phosphorus/day when lanthanum was administered at a dose of 3 g per day with food. By comparison, in one study with an untreated control group (n=10) and another study with a placebo group (n=3), the corresponding mean changes from baseline were 3 mg phosphorus/day and 87 mg phosphorus/day, respectively.
In healthy subjects FOSRENOL Oral Powder was found to be similar to FOSRENOL Chewable Tablets, based on urinary phosphate excretion.
12.3 Pharmacokinetics
Absorption and Distribution - Following single or multiple dose oral administration of FOSRENOL to healthy subjects, the concentration of lanthanum in plasma was very low (bioavailability <0.002%). Following oral administration in patients, the mean lanthanum Cmax was 1.0 ng/mL. During long-term administration (52 weeks) in ESRD patients, the mean lanthanum concentration in plasma was approximately 0.6 ng/mL. There was minimal increase in plasma lanthanum concentrations with increasing doses within the therapeutic dose range. The timing of food intake relative to lanthanum administration (during and 30 minutes after food intake) has a negligible effect on the systemic level of lanthanum.
Systemic exposure to lanthanum was approximately 30% higher following administration of FOSRENOL Oral Powder when compared to FOSRENOL Chewable Tablets. However, systemic exposure to lanthanum from both formulations in this study was within the range seen in previous pharmacokinetic studies of Chewable Tablets in healthy individuals.
In vitro, lanthanum is highly bound (>99%) to human plasma proteins, including human serum albumin, α1-acid glycoprotein, and transferrin. Binding to erythrocytes in vivo is negligible in rats.
In animal studies, lanthanum concentrations in several tissues, particularly gastrointestinal tract, mesenteric lymph nodes, bone and liver, increased over time to levels several orders of magnitude higher than those in plasma. The level of lanthanum in the liver was higher in renally impaired rats due to higher intestinal absorption. Lanthanum was found in the lysosomes and the biliary canal consistent with transcellular transport. Steady state tissue concentrations in bone and liver were achieved in dogs between 4 and 26 weeks. Relatively high levels of lanthanum remained in these tissues for longer than 6 months after cessation of dosing in dogs. There is no evidence from animal studies that lanthanum crosses the blood-brain barrier.
In 105 bone biopsies from patients treated with FOSRENOL for up to 4.5 years, rising levels of lanthanum were noted over time. Estimates of elimination half-life from bone ranged from 2.0 to 3.6 years. Steady state bone concentrations were not reached during the period studied.
Metabolism and Elimination - Lanthanum is not metabolized. Lanthanum was cleared from plasma of patients undergoing dialysis with an elimination half-life of 53 hours following discontinuation of therapy.
No information is available regarding the mass balance of lanthanum in humans after oral administration. In rats and dogs, the mean recovery of lanthanum after an oral dose was about 99% and 94%, respectively, and was essentially all from feces. Biliary excretion is the predominant route of elimination for circulating lanthanum in rats. In healthy volunteers administered intravenous lanthanum as the soluble chloride salt (120 µg), renal clearance was less than 2% of total plasma clearance.
Drug Interactions
FOSRENOL has a low potential for systemic drug-drug interactions because of the very low bioavailability of lanthanum and because it is not a substrate or inhibitor of major cytochrome P450 enzyme groups involved in drug metabolism (CYP1A2, CYP2C9/10, CYP2C19, CYP2D6 and CYP3A4/5).
FOSRENOL does not alter gastric pH. Therefore, FOSRENOL drug interactions based on altered gastric pH are not expected.
In an in vitro investigation, lanthanum did not form insoluble complexes when mixed in simulated gastric fluid with warfarin, digoxin, furosemide, phenytoin, metoprolol and enalapril. Clinical studies have shown that FOSRENOL (three doses of 1000 mg on the day prior to exposure and one dose of 1000 mg on the day of co-administration) administered 30 minutes earlier did not alter the pharmacokinetics of oral warfarin (10 mg), digoxin (0.5 mg), or metoprolol (100 mg). Potential pharmacodynamic interactions between lanthanum and these drugs (e.g., bleeding time or prothrombin time) were not evaluated. None of the drug interaction studies were done with the maximum recommended therapeutic dose of lanthanum carbonate. No drug interaction studies assessed the effects of drugs on phosphate binding by lanthanum carbonate.
Ciprofloxacin
In a randomized, two–way crossover study in healthy volunteers examining the interaction potential of a single oral dose of ciprofloxacin (750 mg) alone and with lanthanum carbonate (1 g TID), the maximum plasma concentration of ciprofloxacin was reduced by 56% and the area under the ciprofloxacin plasma concentration-time curve was reduced by 54%. The 24-h urinary recovery of ciprofloxacin was reduced 52% by FOSRENOL [see Drug Interactions (7.2)].
Levothyroxine
In a single-dose crossover study of levothyroxine (1 mg) with or without simultaneous administration of a single dose of FOSRENOL (500 mg) in six euthyroid normal healthy volunteers, the area under the serum T4 concentration-time curve was decreased by 40% [see Drug Interactions (7.3)].
Fat Soluble Vitamins
FOSRENOL appears not to affect the availability of fat soluble vitamins (A, D, E and K) or other nutrients [see Clinical Studies (14.2)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Oral administration of lanthanum carbonate to rats for up to 104 weeks, at doses up to 1500 mg of the salt per kg/day [2.5 times the MRHD of 5725 mg, on a mg/m2 basis, assuming a 60-kg patient] revealed no evidence of carcinogenic potential. In the mouse, oral administration of lanthanum carbonate for up to 99 weeks, at a dose of 1500 mg/kg/day (1.3 times the MRHD) was associated with an increased incidence of glandular stomach adenomas in male mice.
Lanthanum carbonate tested negative for mutagenic activity in an in vitro Ames assay using Salmonella typhimurium and Escherichia coli strains and in vitro HGPRT gene mutation and chromosomal aberration assays in Chinese hamster ovary cells. Lanthanum carbonate also tested negative in an oral mouse micronucleus assay at doses up to 2000 mg/kg (1.7 times the MRHD), and in micronucleus and unscheduled DNA synthesis assays in rats given IV lanthanum chloride at doses up to 0.1 mg/kg, a dose that produced plasma lanthanum concentrations >2000 times the peak human plasma concentration.
Lanthanum carbonate, at doses up to 2000 mg/kg/day (3.4 times the MRHD), did not affect fertility or mating performance of male or female rats.
13.3 Developmental Toxicity
In pregnant rats, oral administration of lanthanum carbonate at doses as high as 2000 mg/kg/day (3.4 times the MRHD) resulted in no evidence of harm to the fetus. In pregnant rabbits, oral administration of lanthanum carbonate at 1500 mg/kg/day (5 times the MRHD) was associated with a reduction in maternal body weight gain and food consumption, increased post-implantation loss, reduced fetal weights, and delayed fetal ossification. Lanthanum carbonate administered to rats from implantation through lactation at 2000 mg/kg/day (3.4 times the MRHD) caused delayed eye opening, reduction in body weight gain, and delayed sexual development (preputial separation and vaginal opening) of the offspring.
-
14 CLINICAL STUDIES
The effectiveness of FOSRENOL in reducing serum phosphorus in ESRD patients was demonstrated in one short-term, placebo-controlled, double-blind dose-ranging study, two placebo-controlled randomized withdrawal studies and two long-term, active-controlled, open-label studies in both hemodialysis and peritoneal dialysis (PD) patients.
14.1 Double-Blind Placebo-Controlled Studies
One hundred and forty-four patients with chronic renal failure undergoing hemodialysis and with elevated phosphate levels were randomized to double-blind treatment at a fixed dose of lanthanum carbonate of 225 mg (n=27), 675 mg (n=29), 1350 mg (n=30) or 2250 mg (n=26) or placebo (n=32) in divided doses with meals. Fifty-five percent of subjects were male, 71% black, 25% white and 4% of other races. The mean age was 56 years and the duration of dialysis ranged from 0.5 to 15.3 years. Steady-state effects were achieved after two weeks. The effect after six weeks of treatment is shown in Figure 1.
Figure 1. Difference in Phosphate Reduction in the FOSRENOL and Placebo Group in a 6-Week, Dose-Ranging, Double-Blind Study in ESRD Patients (with 95% Confidence Intervals)
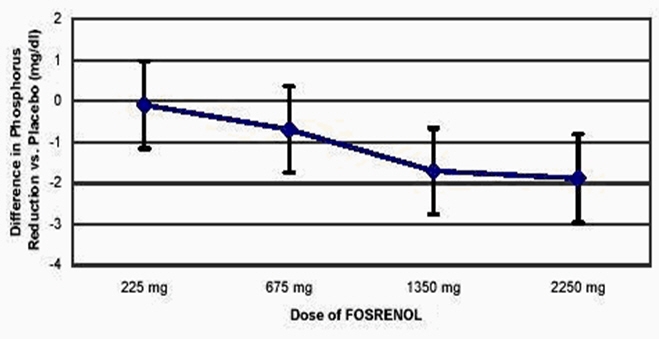
One-hundred and eighty-five patients with end stage renal disease undergoing either hemodialysis (n=146) or peritoneal dialysis (n=39) were enrolled in two placebo-controlled, randomized withdrawal studies. Sixty-four percent of subjects were male, 28% black, 62% white and 10% of other races. The mean age was 58.4 years and the duration of dialysis ranged from 0.2 to 21.4 years. After titration of lanthanum carbonate to achieve a phosphate level between 4.0 and 5.6 mg/dL in one study (doses up to 2250 mg/day) or ≤5.9 mg/dL in the second study (doses up to 3000 mg/day) and maintenance through 6 weeks, patients were randomized to lanthanum or placebo. During the placebo-controlled, randomized withdrawal phase (four weeks), the phosphorus concentration rose in the placebo group by 1.7 mg/dL in one study and 1.9 mg/dL in the other study relative to patients who remained on lanthanum carbonate therapy.
14.2 Open-Label Active-Controlled Studies
Two long-term open-label studies were conducted, involving a total of 2028 patients with ESRD undergoing hemodialysis. Patients were randomized to receive FOSRENOL or alternative phosphate binders for up to six months in one study and two years in the other. The daily FOSRENOL doses, divided and taken with meals, ranged from 375 mg to 3000 mg. Doses were titrated to reduce serum phosphate levels to a target level. The daily doses of the alternative therapy were based on current prescribing information or those commonly utilized. Both treatment groups had similar reductions in serum phosphate of about 1.8 mg/dL. Maintenance of reduction was observed for up to three years in patients treated with FOSRENOL in long-term, open-label extensions.
No effects of FOSRENOL on serum levels of 25-dihydroxy vitamin D3, vitamin A, vitamin B12, vitamin E and vitamin K were observed in patients who were monitored for 6 months.
Paired bone biopsies (at baseline and at one or two years) in 69 patients randomized to either FOSRENOL or calcium carbonate in one study and 99 patients randomized to either FOSRENOL or alternative therapy in a second study showed no differences in the development of mineralization defects between the groups.
Vital status was known for over 2000 patients, 97% of those participating in the clinical program during and after receiving treatment. The adjusted yearly mortality rate (rate/years of observation) for patients treated with FOSRENOL or alternative therapy was 6.6%.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 FOSRENOL Chewable Tablets
FOSRENOL is supplied as a chewable tablet in three dosage strengths for oral administration: 500 mg tablets, 750 mg tablets, and 1000 mg tablets. Each chewable tablet is white to off-white round, flat with a bevelled edge, and debossed on one side with 'S405' and the dosage strength corresponding to the content of elemental lanthanum.
500 mg Patient Pack (2 bottles of 45 tablets, NDC: 54092-252-45, per each patient pack) NDC: 54092-252-90.
750 mg Patient Pack (6 bottles of 15 tablets, NDC: 54092-253-15, per each patient pack) NDC: 54092-253-90.
1000 mg Patient Pack (9 bottles of 10 tablets, NDC: 54092-254-10, per each patient pack) NDC: 54092-254-90.
16.2 FOSRENOL Oral Powder
FOSRENOL Oral Powder is supplied in two dosage strengths for oral administration: 750 mg and 1000 mg. Each 750 mg and 1000 mg stick pack contains 2.1 g and 2.8 g oral powder, respectively, packed in a polyethylene terephthalate/aluminium/polyethylene laminate.
Strength Carton of 10 Stick Packs Patient Pack of 9 Cartons 750 mg NDC: 54092-256-01 NDC: 54092-256-02 1000 mg NDC: 54092-257-01 NDC: 54092-257-02 -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labelling (Medication Guide).
Advise patients to take FOSRENOL with or immediately after meals [see Dosage and Administration (2)].
Instruct patients on concomitant medications that should be dosed apart from FOSRENOL [see Drug Interactions (7)]
Instruct patients who are prescribed FOSRENOL Chewable Tablets to chew or crush tablets completely before swallowing. Emphasize that FOSRENOL Chewable Tablets should not be swallowed intact. Consider crushing FOSRENOL Chewable Tablets completely or prescribing the oral powder formulation for patients with poor dentition or who have difficulty chewing tablets [see Dosage and Administration (2)].
Instruct patients who are prescribed FOSRENOL Oral Powder to sprinkle powder on a small quantity of applesauce or other similar food. Patients should be instructed to consume the entire dose immediately. FOSRENOL Oral Powder is insoluble so no attempt should be made to dissolve the powder in liquid for administration [see Dosage and Administration (2)].
Advise patients who are taking an oral medication where a reduction in the bioavailability of that medication would have a clinically significant effect on its safety or efficacy to separate the dosing of FOSRENOL from the dosing of the affected drug by several hours [see Drug Interactions (7)].
Advise patients to notify their physician that they are taking FOSRENOL prior to an abdominal X-ray or if they have a history of gastrointestinal disease [see Warnings and Precautions (5.1,5.2)].
-
SPL UNCLASSIFIED SECTION
FOSRENOL Chewable Tablets manufactured by Patheon Manufacturing Services LLC,
5900 Martin Luther King Jr. Highway,
Greenville, NC 27834FOSRENOL Oral Powder manufactured by Catalent Germany Schorndorf GmbH,
Steinbeisstrasse 1-2
D-73614 Schorndorf
GermanyManufactured for Shire US Inc., 300 Shire Way, Lexington, MA 02421.
FOSRENOL® is registered in the U.S. Patent and Trademark Office.
© 2018 Shire US Inc.This product is covered by US patents 5,968,976, 7,465,465, 8,980,327 and 9,023,397.
-
MEDICATION GUIDE
MEDICATION GUIDE
FOSRENOL® (foss-wren-all)
(lanthanum carbonate)Read this Medication Guide before you start taking FOSRENOL and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is the most important information I should know about FOSRENOL?
FOSRENOL may cause a bowel blockage, a hole in the bowel or severe constipation, which can be serious, and sometimes lead to surgery or treatment in a hospital.
- You may have a higher risk of bowel blockage, a hole in the bowel or severe constipation if you take FOSRENOL and have:
- a history of surgery, ulcers or cancer in the stomach or bowel
- a history of bowel blockage, or problems resulting in a decreased movement of food through your stomach and bowel (e.g. feeling full quickly after eating or constipation)
- an infection or inflammation of the stomach/bowel (peritonitis)
Do not swallow FOSRENOL Chewable Tablets whole. Chew tablets completely before swallowing. If you can not chew tablets completely, you may crush the tablets thoroughly before swallowing or discuss the oral powder formulation with your healthcare provider.
What is FOSRENOL?
FOSRENOL is a prescription medicine used in people with end stage renal disease (ESRD) to lower the amount of phosphate in the blood.
Who should not take FOSRENOL?
Do not take FOSRENOL if you:
- have blocked bowels
- have severe constipation
FOSRENOL has not been studied in children and adolescents under 18 years of age.
What should I tell my healthcare provider before taking FOSRENOL?
FOSRENOL may not be right for you. Before starting FOSRENOL, tell your healthcare provider if you:
- have a history of surgery, ulcers or cancer in the stomach or bowel
- have a history of a bowel blockage, constipation, or problems resulting in a decreased movement of food through your stomach and bowel especially if you also have diabetes
- have ulcerative colitis, Crohn's disease or an infection or inflammation of the stomach/bowel (peritonitis)
- plan to have an X-ray of your stomach (abdomen)
- have any other medical conditions
- are pregnant, plan to become pregnant, or plan to breastfeed. It is not known if FOSRENOL will harm your unborn baby
Tell your healthcare provider about all of the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take:
- antacids
- antibiotics
- thyroid medicine
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take FOSRENOL?
- Take FOSRENOL exactly as prescribed by your healthcare provider
- Your healthcare provider will tell you how much FOSRENOL to take
- Your healthcare provider may change your dose if needed
- Chewable Tablets - Do not swallow tablets whole. Chew tablets completely before swallowing. If you cannot chew tablets completely, or if you have tooth disease, you may crush the tablets thoroughly before swallowing or discuss the oral powder formulation with your healthcare provider.
- Oral Powder – Sprinkle powder on a small quantity of applesauce or other similar food. Consume the entire dose immediately. FOSRENOL Oral Powder will not dissolve in liquid.
- Take FOSRENOL with or right after meals
- If you take an antacid medicine, take the antacid 2 hours before or 2 hours after you take FOSRENOL
- If you take medicine for your thyroid (levothyroxine), take the thyroid medicine 2 hours before or 2 hours after you take FOSRENOL
- If you take an antibiotic medicine, take the antibiotic 1 hour before or 4 hours after you take FOSRENOL
What are possible or reasonably likely side effects of FOSRENOL?
See "What is the most important information I should know about FOSRENOL?"
The most common side effects of FOSRENOL include:
- nausea
- vomiting
- diarrhea
- stomach pain
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the side effects of FOSRENOL. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store FOSRENOL?
- Store FOSRENOL between 59°F to 86°F (15°C to 30°C).
Keep FOSRENOL and all medicines out of the reach of children.
General information about FOSRENOL
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use FOSRENOL for a condition for which it was not prescribed. Do not give FOSRENOL to other people, even if they have the same condition. It may harm them.
This Medication Guide summarizes the most important information about FOSRENOL. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about FOSRENOL that is written for healthcare professionals.
For more information go to www.FOSRENOL.com or call 1-800-828-2088.
What are the ingredients in FOSRENOL?
Active ingredient: lanthanum carbonate
Inactive ingredients: colloidal silicon dioxide NF, dextrates (hydrated) NF, and magnesium stearate NF.
This Medication Guide has been approved by the US Food and Drug Administration.
© 2018 Shire US Inc.
300 Shire Way, Lexington, MA 02421.
Issued [11/2018]
- You may have a higher risk of bowel blockage, a hole in the bowel or severe constipation if you take FOSRENOL and have:
-
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Label
NDC: 54092-252-45
FOSRENOL®
(lanthanum carbonate)
chewable tablets500 mg
Do not swallow tablets whole.
Chew or crush tablets completely
before swallowing.
Take with or immediately after meals.ATTENTION PHARMACIST: Each patient is required
to receive the enclosed Medication Guide.45 TABLETS
Rx only
Shire
-
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Carton
NDC: 54092-252-90
FOSRENOL®
(lanthanum carbonate)
chewable tablets500 mg
Do not swallow tablets whole.
Chew or crush tablets completely
before swallowing.
Take with or immediately after
meals.2 bottles/45 chewable tablets each
Rx only
Shire
Active Ingredient: Each
tablet contains 500 mg
of lanthanum as lanthanum
carbonate hydrateInactive Ingredients:
Colloidal silicon dioxide,
dextrates, and magnesium
stearate.See package insert for full
prescribing information.
Store at 25°C (77°F)
excursions permitted to
15-30°C (59-86°F)
[see USP Controlled Room
Temperature]
-
PRINCIPAL DISPLAY PANEL - 750 mg Tablet Bottle Label
NDC: 54092-253-15
FOSRENOL®
(lanthanum carbonate)
chewable tablets750 mg
Do not swallow tablets whole.
Chew or crush tablets completely
before swallowing.
Take with or immediately after meals.ATTENTION PHARMACIST: Each patient is required
to receive the enclosed Medication Guide.15 TABLETS
Rx only
Shire
-
PRINCIPAL DISPLAY PANEL - 750 mg Tablet Bottle Carton
NDC: 54092-253-90
FOSRENOL®
(lanthanum carbonate)
chewable tablets750 mg
Do not swallow tablets whole.
Chew or crush tablets completely
before swallowing.
Take with or immediately after meals.6 bottles/15 chewable tablets each
Rx only
Shire
Active Ingredient: Each
tablet contains 750 mg
of lanthanum as lanthanum
carbonate hydrateInactive Ingredients:
Colloidal silicon dioxide,
dextrates, and magnesium
stearate.See package insert for full
prescribing information.
Store at 25°C (77°F)
excursions permitted to
15-30°C (59-86°F)
[see USP Controlled Room
Temperature]
-
PRINCIPAL DISPLAY PANEL - 1000 mg Tablet Bottle Label
NDC: 54092-254-10
FOSRENOL®
(lanthanum carbonate)
chewable tablets1000 mg
Do not swallow tablets whole.
Chew or crush tablets completely
before swallowing.
Take with or immediately after meals.ATTENTION PHARMACIST: Each patient is required
to receive the enclosed Medication Guide.10 TABLETS
Rx only
Shire
-
PRINCIPAL DISPLAY PANEL - 1000 mg Tablet Bottle Carton
NDC: 54092-254-90
FOSRENOL®
(lanthanum carbonate)
chewable tablets1000 mg
Do not swallow tablets whole.
Chew or crush tablets completely
before swallowing.
Take with or immediately after meals.9 bottles/10 chewable tablets each
Rx only
Shire
Active Ingredient: Each
tablet contains 1000 mg
of lanthanum as lanthanum
carbonate hydrateInactive Ingredients:
Colloidal silicon dioxide,
dextrates, and magnesium
stearate.See package insert for full
prescribing information.
Store at 25° C (77°F)
excursions permitted to
15-30°C (59-86°F)
[see USP Controlled Room
Temperature]
-
PRINCIPAL DISPLAY PANEL - 750 mg Packet Carton - 10 Stick Pack
NDC: 54092-256-01
Fosrenol®
(lanthanum carbonate)
oral powder750 mg
Mix with small quantity of soft food
and take immediatelyShire
10 stick packs
Rx only

-
PRINCIPAL DISPLAY PANEL - 750 mg Packet Carton - 90 Stick Pack
NDC: 54092-256-02
Fosrenol®
(lanthanum carbonate)
oral powder750 mg
Mix with small quantity of soft food
and take immediatelyShire
9 cartons, each carton contains 10 stick packs
Rx only
-
PRINCIPAL DISPLAY PANEL - 1000 mg Packet Carton - 10 Stick Pack
NDC: 54092-257-01
Fosrenol®
(lanthanum carbonate)
oral powder1000 mg
Mix with small quantity of soft food
and take immediatelyShire
10 stick packs
Rx only

-
PRINCIPAL DISPLAY PANEL - 1000 mg Packet Carton - 90 Stick Pack
NDC: 54092-257-02
Fosrenol®
(lanthanum carbonate)
oral powder1000 mg
Mix with small quantity of soft food
and take immediatelyShire
9 cartons, each carton contains 10 stick packs
Rx only
-
INGREDIENTS AND APPEARANCE
FOSRENOL
lanthanum carbonate tablet, chewableProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 54092-252 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANTHANUM CARBONATE (UNII: 490D9F069T) (LANTHANUM CATION (3+) - UNII:O7FU5X12W5) LANTHANUM CATION (3+) 500 mg Inactive Ingredients Ingredient Name Strength DEXTRATES (UNII: G263MI44RU) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (white) Score no score Shape ROUND (ROUND) Size 18mm Flavor Imprint Code S405;500 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54092-252-90 2 in 1 PACKAGE 10/26/2004 1 NDC: 54092-252-45 45 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021468 10/26/2004 FOSRENOL
lanthanum carbonate tablet, chewableProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 54092-253 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANTHANUM CARBONATE (UNII: 490D9F069T) (LANTHANUM CATION (3+) - UNII:O7FU5X12W5) LANTHANUM CATION (3+) 750 mg Inactive Ingredients Ingredient Name Strength DEXTRATES (UNII: G263MI44RU) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (white) Score no score Shape ROUND (ROUND) Size 20mm Flavor Imprint Code S405;750 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54092-253-90 6 in 1 PACKAGE 11/23/2005 1 NDC: 54092-253-15 15 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021468 11/23/2005 FOSRENOL
lanthanum carbonate tablet, chewableProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 54092-254 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANTHANUM CARBONATE (UNII: 490D9F069T) (LANTHANUM CATION (3+) - UNII:O7FU5X12W5) LANTHANUM CATION (3+) 1000 mg Inactive Ingredients Ingredient Name Strength DEXTRATES (UNII: G263MI44RU) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (white) Score no score Shape ROUND (ROUND) Size 22mm Flavor Imprint Code S405;1000 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54092-254-90 9 in 1 PACKAGE 11/23/2005 1 NDC: 54092-254-10 10 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021468 11/23/2005 FOSRENOL
lanthanum carbonate powderProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 54092-256 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANTHANUM CARBONATE (UNII: 490D9F069T) (LANTHANUM CATION (3+) - UNII:O7FU5X12W5) LANTHANUM CATION (3+) 750 mg Inactive Ingredients Ingredient Name Strength DEXTRATES (UNII: G263MI44RU) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54092-256-02 9 in 1 PACKAGE 09/24/2014 1 NDC: 54092-256-01 10 in 1 CARTON; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204734 09/24/2014 FOSRENOL
lanthanum carbonate powderProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 54092-257 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANTHANUM CARBONATE (UNII: 490D9F069T) (LANTHANUM CATION (3+) - UNII:O7FU5X12W5) LANTHANUM CATION (3+) 1000 mg Inactive Ingredients Ingredient Name Strength DEXTRATES (UNII: G263MI44RU) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54092-257-02 9 in 1 CARTON 09/24/2014 1 NDC: 54092-257-01 10 in 1 CARTON; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA204734 09/24/2014 Labeler - Shire US Manufacturing Inc. (964907406) Establishment Name Address ID/FEI Business Operations Patheon Manufacturing Services LLC 079415560 ANALYSIS(54092-252, 54092-253, 54092-254) , LABEL(54092-252, 54092-253, 54092-254) , MANUFACTURE(54092-252, 54092-253, 54092-254) , PACK(54092-252, 54092-253, 54092-254) Establishment Name Address ID/FEI Business Operations Corden Pharma Bergamo SpA 438743601 ANALYSIS(54092-252, 54092-253, 54092-254, 54092-256, 54092-257) , API MANUFACTURE(54092-252, 54092-253, 54092-254, 54092-256, 54092-257) Establishment Name Address ID/FEI Business Operations Element Materials Technology Pharma US LLC 081315325 ANALYSIS(54092-252, 54092-253, 54092-254, 54092-256, 54092-257) Establishment Name Address ID/FEI Business Operations Reading Scientific Services Ltd (RSSL) 293610085 ANALYSIS(54092-252, 54092-253, 54092-254, 54092-256, 54092-257) Establishment Name Address ID/FEI Business Operations Triclinic Labs 017662237 ANALYSIS(54092-252, 54092-253, 54092-254, 54092-256, 54092-257) Establishment Name Address ID/FEI Business Operations Microbac Laboratories, Inc. 178463126 ANALYSIS(54092-252, 54092-253, 54092-254, 54092-256, 54092-257) Establishment Name Address ID/FEI Business Operations Catalent Germany Schorndorf GmbH 315732628 ANALYSIS(54092-256, 54092-257) , LABEL(54092-256, 54092-257) , MANUFACTURE(54092-256, 54092-257) , PACK(54092-256, 54092-257) Establishment Name Address ID/FEI Business Operations BCM Ltd 230780322 ANALYSIS(54092-256, 54092-257) Establishment Name Address ID/FEI Business Operations Reckitt Benckiser Healthcare International Ltd. 230780363 ANALYSIS(54092-252, 54092-253, 54092-254) , MANUFACTURE(54092-252, 54092-253, 54092-254)
Trademark Results [Fosrenol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 FOSRENOL 77685009 3685795 Dead/Cancelled |
Shire International Licensing BV 2009-03-06 |
 FOSRENOL 76118066 2687871 Live/Registered |
Shire International Licensing BV 2000-08-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.