EQUICOXIB- firocoxib solution
EquiCoxib by
Drug Labeling and Warnings
EquiCoxib by is a Animal medication manufactured, distributed, or labeled by Aurora Pharmaceutical, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
Description:
EquiCoxib (firocoxib) belongs to the coxib class of non-narcotic, non-steroidal anti-inflammatory drugs (NSAIDs). Firocoxib is a white crystalline compound described chemically as 3-(cyclopropylmethoxy)-4-(4-(methylsulfonyl)phenyl)-5,5-dimethylfuranone. The empirical formula is C17H20O5S, and the molecular weight is 336.4. The structural formula is shown below:
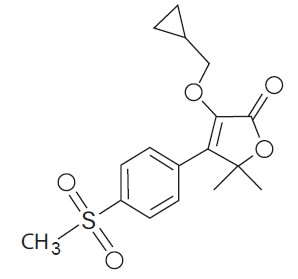
- Indications:
-
Dosage and Administration:
Always provide the Client Information Sheet with the prescription. The recommended dosage of EquiCoxib (firocoxib) for oral administration in horses is 0.045 mg/lb (0.1 mg/kg) of body weight once daily for up to 14 days. In target animal safety studies, toxicity was seen at the recommended dose when the duration of treatment exceeded 30 days. Only administer EquiCoxib with the provided dosing syringe.
Each 1.25 mL volume will treat 250 pounds of body weight and each additional 0.25 mL volume corresponds to approximately a 50 lb weight increment. The provided dosing syringe is calibrated so that each line corresponds to a 50 lb weight increment. To deliver the correct dose, round the horse's body weight up to the nearest 50 pound increment (if body weight is an exact 50 pound increment, do not round up).
-
FOR ORAL USE ONLY. DO NOT INJECT EQUICOXIB. ONLY ADMINISTER WITH THE PROVIDED DOSING SYRINGE.
EquiCoxib Oral Dosing Guide Body Weight (lb) Dose Volume (mL) 250 1.25 mL 500 2.5 mL 750 3.75 mL 1000 5 mL 1250 6.25 mL 1) Remove draw-off cap. Peel off the foil-backed seal from the bottle.
2) Screw the draw-off cap tightly back on the bottle.
3) Remove the seal from the top of the cap exposing the cross-hatched opening in the center of the silicone liner.
4) Remove the provided oral dosing syringe from its plastic cover.
5) Insert the oral dosing syringe firmly into the cross-hatched opening of the caps silicone liner.
6) Turn the bottle with attached syringe upside down. Pull back the syringe plunger until the widest portion of the plunger lines up with the line that corresponds with the animals weight. Each line between the 250 lb increments corresponds to 50 lb.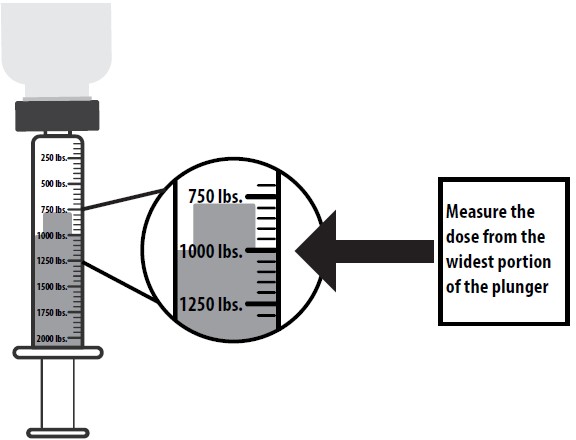
7) Turn the bottle with attached syringe right side up and separate the dosing syringe from the bottle.
8) Give orally according to your veterinarians instructions.
DO NOT INJECT. - Contraindications:
-
Warnings:
For oral use in horses only. Do not use in horses intended for human consumption.
Human Warnings: Not for use in humans. Keep this and all medications out of the reach of children. Wash hands with soap and water after use. Consult a physician in case of accidental ingestion by humans.
Animal Safety: Clients should be advised to observe for signs of potential drug toxicity and be given a Client Information Sheet with each prescription.
Keep EquiCoxib in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Aurora Pharmaceutical at 1-888-215-1256 or www.aurorapharmaceutical.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
-
Precautions:
Horses should undergo a thorough history and physical examination before initiation of NSAID therapy. Appropriate laboratory tests should be conducted to establish hematological and serum biochemical baseline data before and periodically during administration of any NSAID. Clients should be advised to observe for signs of potential drug toxicity and be given a Client Information Sheet with each prescription. See Information for Owner or Person Treating Horse section of this package insert.
Treatment with EquiCoxib should be terminated if signs such as inappetance, colic, abnormal feces, or lethargy are observed. As a class, cyclooxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal, and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Horses that have experienced adverse reactions from one NSAID may experience adverse reactions from another NSAID. Patients at greatest risk for adverse events are those that are dehydrated, on diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction. Concurrent administration of potentially nephrotoxic drugs should be carefully approached or avoided. NSAIDs may inhibit the prostaglandins that maintain normal homeostatic function. Such anti-prostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that has not been previously diagnosed. Since many NSAIDs possess the potential to produce gastrointestinal ulcerations and/or gastrointestinal perforation, concomitant use of EquiCoxib Oral Solution with other anti-inflammatory drugs, such as NSAIDs or corticosteroids, should be avoided. The concomitant use of protein bound drugs with EquiCoxib Oral Solution has not been studied in horses. The influence of concomitant drugs that may inhibit the metabolism of EquiCoxib Oral Solution has not been evaluated. Drug compatibility should be monitored in patients requiring adjunctive therapy. The safe use of EquiCoxib Oral Solution in horses less than one year in age, horses used for breeding, or in pregnant or lactating mares has not been evaluated. Consider appropriate washout times when switching from one NSAID to another NSAID or corticosteroid.
-
Adverse Reactions:
In controlled field studies, 127 horses (ages 3 to 37 years) were evaluated for safety when given firocoxib at a dose of 0.045 mg/lb (0.1 mg/kg) orally once daily for up to 14 days. The following adverse reactions were observed. Horses may have experienced more than one of the observed adverse reactions during the study.
Adverse Reactions Seen in U.S. Field Studies
Firocoxib was safely used concomitantly with other therapies, including vaccines, anthelmintics, and antibiotics, during the field studies. The safety data sheet (SDS) contains more detailed occupational safety information.
To report suspected adverse drug events, for technical assistance, or to obtain a copy of the Safety Data Sheet (SDS), contact Aurora Pharmaceutical Inc. at 1-888-215-1256 or www.aurorapharmaceutical.com. For additional
information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or online at www.fda.gov/reportanimalae.Adverse Reactions Firocoxib
n=127Active Control
n=125Abdominal pain
0
1
Diarrhea
2
0
Excitation
1
0
Lethargy
0
1
Loose stool
1
0
Polydipsia
0
1
Urticaria
0
1
-
Information for Owner or Person Treating Horse:
You should give a Client Information Sheet to the person treating the horse and advise them of the potential for adverse reactions and the clinical signs associated with NSAID intolerance. Adverse reactions may include erosions and ulcers of the gums, tongue, lips and face, weight loss, colic, diarrhea, or icterus. Serious adverse reactions associated with this drug class can occur without warning and, in some situations, result in death. Clients should be advised to discontinue NSAID therapy and contact their veterinarian immediately if any of these signs of intolerance are observed. The majority of patients with drug-related adverse reactions recover when the signs are recognized, drug administration is stopped, and veterinary care is initiated.
-
Clinical Pharmacokinetics / Pharmacodynamics:
Pharmacokinetics: When administered as a 0.045 mg/lb (0.1 mg/kg) dose in oral paste to adult horses with normal access to roughage, feed, and water, the absolute bioavailability of firocoxib from oral paste is approximately 79%. Following oral administration, drug peak concentration (Cmax) of 0.08 mcg/mL can be reached at 4 hours (Tmax) post-dosing. However, in some animals, up to 12 hours may be needed before significant plasma concentrations are observed. Little drug amount distributes into blood cells. The major metabolism mechanism of firocoxib in the horse is decyclopropylmethylation followed by glucuronidation of that metabolite. Based upon radiolabel studies, the majority of firocoxib is eliminated in the urine as the decyclopropylmethylated metabolite. Despite a high rate of plasma protein binding (98%), firocoxib exhibits a large volume of distribution (mean Vd(ss) = 1652 mL/kg). The terminal elimination half-life (T1/2) in plasma averages 30-40 hours after IV or oral paste dosing. Therefore, drug accumulation occurs with repeated dose administrations and steady state concentrations are achieved beyond
6-8 daily oral doses in the horse. Dose linearity exists from 1X-2X of 0.1 mg/kg/day.Mode of action: EquiCoxib (firocoxib) is a cyclooxygenase-inhibiting (coxib) class, non-narcotic, non-steroidal anti-inflammatory drug (NSAID) with anti-inflammatory, analgesic and antipyretic activity1 in animal models. Based on in vitro horse data, firocoxib is a selective inhibitor of prostaglandin biosynthesis through inhibition of inducible cyclooxygenase-2-isoenzyme (COX-2)2. Firocoxib selectivity for the constitutive isoenzyme, cyclooxygenase-1 (COX-1) is relatively low. However, the clinical significance of these in vitro selectivity findings has not been established.
-
Effectiveness:
Two hundred fifty-three client-owned horses of various breeds, ranging in age from 2 to 37 years and weighing from 595 to 1638 lbs, were randomly administered firocoxib oral paste or an active control drug in multi-center field studies. Two hundred forty horses were evaluated for effectiveness and 252 horses were evaluated for safety. Horses were assessed for lameness, pain on manipulation, range of motion, joint swelling, and overall clinical improvement in a non-inferiority evaluation of firocoxib oral paste compared to an active control. At study’s end, 84.4% of horses treated with firocoxib oral paste were judged improved on veterinarians’ clinical assessment, and 73.8% were also rated improved by owners.
Horses treated with firocoxib oral paste showed improvement in veterinarian-assessed lameness, pain on manipulation, range of motion, and joint swelling that was comparable to the active control. -
Animal Safety:
In a target animal safety study, firocoxib was administered orally to healthy adult horses (two male castrates and four females per group) at 0, 0.1, 0.3 and 0.5 mg firocoxib/kg body weight (1, 3 and 5X the recommended dose) for 30 days. Administration of firocoxib at 0.3 and 0.5 mg/kg body weight was associated with an increased incidence of oral ulcers as compared to the control group but, no oral ulcers were noted with 0.1 mg/kg. There were no other drug-related adverse findings in this study.
In another target animal safety study, firocoxib was administered orally to healthy adult horses (four males or male castrates and four females per group) at 0, 0.1, 0.3 and 0.5 mg firocoxib/kg body weight (1, 3 and 5X the recommended dose) for 42 days. Administration of firocoxib at 0.1, 0.3 and 0.5 mg/kg body weight was associated with delayed healing of pre-existing oral (lip, tongue, gingival) ulcers. In addition, the incidence of oral ulcers was higher in all treated groups as compared to the control group.
Clinical chemistry and coagulation abnormalities were seen in several horses in the 0.5 mg/kg (5X) group. One 5X male horse developed a mildly elevated BUN and creatinine over the course of the study, prolonged buccal mucosal bleeding time (BMBT), and a dilated pelvis of the right kidney. Another 5X male had a similar mild increase in creatinine during the study but did not have any gross abnormal findings. One female in the 5X group had a prolonged BMBT, bilateral tubulointerstitial nephropathy and bilateral papillary necrosis. Tubulointerstitial nephropathy occurred in one 3X female, two 3X male horses, and the 5X female horse discussed above with the prolonged BMBT. Papillary necrosis was present in one 1X male horse and the 5X female horse discussed above. Despite the gross and microscopic renal lesions, all of the horses were clinically healthy and had normal hematology, clinical chemistry and urinalysis values.
In another target animal safety study, firocoxib was administered orally to healthy adult horses (three females, two male castrates and one male per group) at 0, 0.25 mg/kg, 0.75 mg/kg and 1.25 mg/kg (2.5, 7.5 and 12.5X the recommended dose of 0.1 mg/kg) for 92 days. An additional group of three females, two male castrates and one male per group, was dosed at 1.25 mg/kg for 92 days but was monitored until Days 147-149. There were treatment-related adverse events in all treated groups. These consisted of ulcers of the lips, gingiva and tongue and erosions of the skin of the mandible and head. Gross and microscopic lesions of the kidneys consistent with tubulointerstitial nephropathy were seen in all treated groups. Papillary necrosis was seen in the 2.5X and 12.5X groups. In addition, several 12.5X horses had elevated liver enzymes (GGT, SDH, AST and ALT). One 2.5X horse had increased urine GGT and urine protein levels which was due to renal hemorrhage and nephropathy. Gastric ulcers of the margo plicatus and glandular area were more prevalent in the 2.5X and 7.5X groups, but not seen in the 12.5X group. The group of horses that were monitored until Days 147-149 showed partial to full recovery from oral and skin ulcers, but no recovery from tubulointerstitial nephropathy.
- Storage Information:
- How Supplied:
-
References:
1McCann ME, Rickes EL, Hora DF, Cunningham PK et al. In vitro effects and in vivo efficacy of a novel cyclooxygenase-2 inhibitor in cats with lipopolysaccharide-induced pyrexia. Am J Vet Res. 2005 Jul;66 (7):1278-84
2McCann ME, Anderson DR, Brideau C et al. In vitro activity and in vivo efficacy of a novel COX-2 inhibitor in the horse. Proceedings of the Academy of Veterinary Internal Medicine. 2002. Abstract 114, p.789.
- SPL UNCLASSIFIED SECTION
-
Client Information Sheet
EquiCoxib™
(firocoxib)
Oral Solution for Horses
Non-steroidal anti-inflammatory drug
Information for Horse Owners
EquiCoxib™ Oral Solution is administered for up to 14 days for the control of pain and inflammation associated with osteoarthritis in horses.
This summary contains important information about EquiCoxib. You should read this information before you start giving your horse EquiCoxib Oral Solution and review it each time your prescription is refilled. This sheet is provided only as a summary and does not take the place of instructions from your veterinarian. Talk to your veterinarian if you do not understand any of this information or you want to know more about EquiCoxib.
What is EquiCoxib?
EquiCoxib is a veterinary prescription non-steroidal anti-inflammatory drug (NSAID) of the coxib class used to control pain and inflammation associated with osteoarthritis in horses. Osteoarthritis (OA) is a painful condition caused by progressive "wear and tear" of cartilage and other parts of the joints that may result in the following changes or signs in your horse:
- Limping or lameness.
- Decreased activity or exercise (reluctance to stand, walk, trot or run, or difficulty in performing these activities).
- Stiffness or decreased movement of joints.
How to give EquiCoxib to your horse.
EquiCoxib should be given according to your veterinarian's instructions. Do not change the way you give EquiCoxib to your horse without first speaking with your veterinarian.
Do not exceed 14 days of treatment.
The recommended dosage of EquiCoxib (firocoxib) for oral administration in horses is 0.045 mg/lb (0.1 mg/kg) of body weight once daily for up to 14 days.
Each 1.25 mL volume will treat 250 pounds of body weight and each additional 0.25 mL corresponds to 50 lb weight increment. To deliver the correct dose, round the horse's body weight up to the nearest 50 pound increment (if the body weight is an exact 50 pound increment, do not round up). EquiCoxib may be given with or without food.What kind of results can I expect when my horse is on EquiCoxib for OA?
While EquiCoxib is not a cure for osteoarthritis, it can control the pain and inflammation associated with OA and can improve your horse's mobility.
- Response varies from horse to horse, but improvement can be quite dramatic.
- Improvement can be seen in just a few hours in most horses.
Which horses should not receive EquiCoxib?
Your horse should not be given EquiCoxib if he/she:
- Has an allergic reaction to firocoxib, the active ingredient in EquiCoxib.
- Has previously had an allergic reaction (such as hives, facial or lower limb swelling, or red or itchy skin) to aspirin or other NSAIDs.
- Is presently taking aspirin, phenylbutazone, flunixin meglumine, diclofenac, ketoprofen, or other NSAIDs or corticosteroids.
- The safety of EquiCoxib has not been determined in horses less than one year of age or in breeding horses, pregnant or lactating mares.
EquiCoxib Oral Solution should only be given orally to horses.
- EquiCoxib is not for use in horses intended for human food consumption.
- People should not take EquiCoxib. Keep EquiCoxib and all medications out of the reach of children. Consult a physician in case of accidental ingestion by humans.
What to tell/ask your veterinarian before giving EquiCoxib.
Talk to your veterinarian about:
- The signs of OA you have observed in your horse, such as limping or stiffness.
- If any tests, such as X-rays, will be done before EquiCoxib is prescribed.
- How often your horse may need to be examined by your veterinarian.
- The risks and benefits of using EquiCoxib.
Tell your veterinarian if your horse has ever had the following medical problems:
- Any side effects from taking EquiCoxib or other NSAIDs, such as aspirin or phenylbutazone.
- Any kidney disease.
- Any liver disease.
- Any gastrointestinal ulcers.
Tell your veterinarian about:
- Other medical problems or allergies that your horse has now, or has had in the past.
- All medicines that you are giving or plan to give to your horse, including those you can get without a prescription and any dietary supplements.
Tell your veterinarian if you plan to breed your horse, or if your mare is pregnant or nursing a foal.
What are the possible side effects that may occur in my horse during EquiCoxib therapy?
EquiCoxib, like other NSAIDS, may cause some side effects. Serious side effects associated with NSAID therapy in horses can occur with or without warning. The most common side effects associated with EquiCoxib therapy involve the tongue, lips and skin of the mouth and face (erosions and ulcers of the mucosa and skin) and the kidney. Gastrointestinal, kidney and liver problems have also been reported with other NSAIDs. Look for the following side effects that may indicate your horse is having a problem with EquiCoxib or may have another medical problem:
- Sores or ulcers on the tongue and inside of mouth.
- Sores, scabs, redness, or rubbing of the facial skin, particularly around the mouth.
- Change in eating or drinking habits (frequency or amount consumed).
- Change in urination habits (frequency or color).
- Yellowing of gums, skin, or whites of the eyes (jaundice).
- Unexpected weight loss.
- Change in behavior (such as increased or decreased activity level).
It is important to stop therapy and contact your veterinarian if you think your horse has a medical problem or side effect while taking EquiCoxib Oral Solution. If you have additional questions about possible side effects, talk with your veterinarian or call 1-888-215-1256.
Can EquiCoxib be given with other medications?
EquiCoxib should not be given with other NSAIDs (for example, aspirin, phenylbutazone, diclofenac, ketoprofen or flunixin) or systemic corticosteroids (for example, prednisone, cortisone, dexamethasone, or triamcinolone). Tell your veterinarian about all medications that you have given your horse in the past, and any medications you are planning to give with EquiCoxib Oral Solution. This should include other medicines that you can get without a prescription or any dietary supplements. Your veterinarian may want to check that all of your horse's medicines can be given together.
What do I do in case my horse receives more than the prescribed amount of EquiCoxib?
- Consult your veterinarian if your horse receives more than the prescribed amount of EquiCoxib.
What else should I know about EquiCoxib?
- This sheet provides a summary of information about EquiCoxib Oral Solution and general information about NSAIDs. If you have any questions or concerns about EquiCoxib or osteoarthritis pain, talk with your veterinarian.
- As with all prescribed medicines, EquiCoxib Oral Solution should only be given to the horse for which it is prescribed. It should be given to your horse only for the condition for which it is prescribed, at the labeled dose and duration.
- It is important to periodically discuss your horse's response to EquiCoxib Oral Solution. Your veterinarian will determine if your horse is responding as expected and if your horse should continue receiving EquiCoxib Oral Solution.
DOSAGE AND ADMINISTRATION:
Always provide the Client Information Sheet with the prescription. The recommended dosage of EquiCoxib (firocoxib) for oral administration in horses is 0.045 mg/lb (0.1 mg/kg) of body weight once daily for up to 14 days. In target animal safety studies, toxicity was seen at the recommended dose when the duration of treatment exceeded 30 days. Only administer EquiCoxib with the provided dosing syringe.
Each 1.25 mL volume will treat 250 pounds of body weight and each additional 0.25 mL volume corresponds to approximately a 50 lb weight increment. The provided dosing syringe is calibrated so that each line corresponds to a 50 lb weight increment. To deliver the correct dose, round the horse's body weight up to the nearest 50 pound increment (if body weight is an exact 50 pound increment, do not round up).
FOR ORAL USE ONLY. DO NOT INJECT EQUICOXIB.
ONLY ADMINISTER WITH THE PROVIDED
DOSING SYRINGE.
EquiCoxib Oral Dosing Guide Body Weight (lb) Dose Volume (mL) 250 1.25 mL 500 2.5 mL 750 3.75 mL 1000 5 mL 1250 6.25 mL 1) Remove draw-off cap. Peel off the foil-backed seal from the bottle.
2) Screw the draw-off cap tightly back on the bottle.
3) Remove the seal from the top of the cap exposing the cross-hatched opening in the center of the silicone liner.
4) Remove the provided oral dosing syringe from its plastic cover.
5) Insert the oral dosing syringe firmly into the cross-hatched
opening of the caps silicone liner.
6) Turn the bottle with attached syringe upside down. Pull back the syringe plunger until the widest portion of the plunger lines up with the line that corresponds with the animals weight. Each line between the 250 lb increments corresponds to 50 lb.
7) Turn the bottle with attached syringe right side up and separate the dosing syringe from the bottle.
8) Give orally according to your veterinarians instructions.
DO NOT INJECT. -
Principal Display Panel – 90mL Bottle Carton
Approved by FDA under ANADA # 200-766
EquiCoxib™
(firocoxib)Non-steroidal anti-inflammatory for the control of pain and inflammation associated with osteoarthritis in horses.
Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Net Contents: 90 mL
Oral Solution for HorsesIN 40-1755 11/2024

-
INGREDIENTS AND APPEARANCE
EQUICOXIB
firocoxib solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 51072-116 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength firocoxib (UNII: Y6V2W4S4WT) (firocoxib - UNII:Y6V2W4S4WT) firocoxib 9.0 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51072-116-00 1 in 1 CARTON 1 90 mL in 1 BOTTLE 2 NDC: 51072-116-01 1 in 1 CARTON 2 400 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200766 03/01/2024 Labeler - Aurora Pharmaceutical, Inc. (832848639) Establishment Name Address ID/FEI Business Operations Aurora Pharmaceutical, Inc. 832848639 manufacture
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.