PROGRAF- tacrolimus capsule, gelatin coated
Prograf by
Drug Labeling and Warnings
Prograf by is a Prescription medication manufactured, distributed, or labeled by Rebel Distributors Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
WARNING
Increased susceptibility to infection and the possible development of lymphoma may result from immunosuppression. Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should prescribe Prograf. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient.
-
DESCRIPTION
Prograf is available for oral administration as capsules (tacrolimus capsules) containing the equivalent of 0.5 mg, 1 mg or 5 mg of anhydrous tacrolimus. Inactive ingredients include lactose, hydroxypropyl methylcellulose, croscarmellose sodium, and magnesium stearate. The 0.5 mg capsule shell contains gelatin, titanium dioxide and ferric oxide, the 1 mg capsule shell contains gelatin and titanium dioxide, and the 5 mg capsule shell contains gelatin, titanium dioxide and ferric oxide.
Prograf is also available as a sterile solution (tacrolimus injection) containing the equivalent of 5 mg anhydrous tacrolimus in 1 mL for administration by intravenous infusion only. Each mL contains polyoxyl 60 hydrogenated castor oil (HCO-60), 200 mg, and dehydrated alcohol, USP, 80.0% v/v. Prograf injection must be diluted with 0.9% Sodium Chloride Injection or 5% Dextrose Injection before use.
Tacrolimus, previously known as FK506, is the active ingredient in Prograf. Tacrolimus is a macrolide immunosuppressant produced by Streptomyces tsukubaensis. Chemically, tacrolimus is designated as [3S-[3R*[E(1S*,3S*,4S*)], 4S*,5R*,8S*,9E,12R*,14R*,15S*,16R*,18S*,19S*,26aR*]] -5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(2-propenyl)-15,19-epoxy-3H-pyrido[2,1-c][1,4] oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone, monohydrate.
The chemical structure of tacrolimus is:
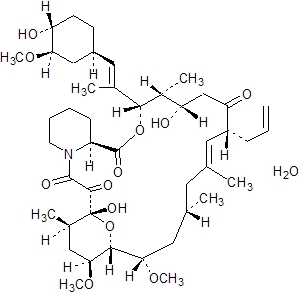
Tacrolimus has an empirical formula of C44H69NO12H2O and a formula weight of 822.03. Tacrolimus appears as white crystals or crystalline powder. It is practically insoluble in water, freely soluble in ethanol, and very soluble in methanol and chloroform.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Tacrolimus prolongs the survival of the host and transplanted graft in animal transplant models of liver, kidney, heart, bone marrow, small bowel and pancreas, lung and trachea, skin, cornea, and limb.
In animals, tacrolimus has been demonstrated to suppress some humoral immunity and, to a greater extent, cell-mediated reactions such as allograft rejection, delayed type hypersensitivity, collagen-induced arthritis, experimental allergic encephalomyelitis, and graft versus host disease.
Tacrolimus inhibits T-lymphocyte activation, although the exact mechanism of action is not known. Experimental evidence suggests that tacrolimus binds to an intracellular protein, FKBP-12. A complex of tacrolimus-FKBP-12, calcium, calmodulin, and calcineurin is then formed and the phosphatase activity of calcineurin inhibited. This effect may prevent the dephosphorylation and translocation of nuclear factor of activated T-cells (NF-AT), a nuclear component thought to initiate gene transcription for the formation of lymphokines (such as interleukin-2, gamma interferon). The net result is the inhibition of T-lymphocyte activation (i.e., immunosuppression).
Pharmacokinetics
Tacrolimus activity is primarily due to the parent drug. The pharmacokinetic parameters (mean±S.D.) of tacrolimus have been determined following intravenous (IV) and/or oral (PO) administration in healthy volunteers, and in kidney transplant, liver transplant, and heart transplant patients. (See table below.)
- * not applicable
- † AUC0-120;
- ‡ AUC0-72
- § Corrected for individual bioavailability
- ¶ AUC0-inf;
- # not available
- Þ AUC0-t;
- ß Determined after the first dose
- à Median [range]
- è AUC0-12
Population
N
Route
(Dose)
Parameters Cmax
(ng/mL)Tmax
(hr)AUC
(nghr/mL)t1/2
(hr)CI
(L/hr/kg)V
(L/kg)
Healthy
Volunteers
8
IV
(0.025 mg/kg/4hr)
598†
± 125
34.2
± 7.7
0.040
± 0.009
1.91
± 0.31
16 PO
(5 mg)
29.7
± 7.2
1.6
± 0.7
243‡
± 73
34.8
± 11.40.041 §
± 0.0081.94§
± 0.53
Kidney
Transplant
Pts
26
IV
(0.02 mg/kg/12 hr)* * 294¶
± 262
18.8
± 16.70.083
± 0.0501.41
± 0.66PO
(0.2 mg/kg/day)19.2
± 10.33.0
203¶
± 42
# # # PO
(0.3 mg/kg/day)24.2
± 15.8
1.5
288¶
± 93# # #
Liver
Transplant
Pts17
IV
(0.05 mg/kg/12 hr)3300¶
± 2130
11.7
± 3.90.053
± 0.017
0.85
± 0.30PO
(0.3 mg/kg/day)68.5
± 30.02.3
± 1.5519¶
± 179# # #
Heart
Transplant Patients
11 IV
(0.01 mg/kg/day as a continuous infusion)* * 954Þ
±33423.6
±9.220.051
±0.015# 11 PO
(0.075mg/kg/day)ß14.7+7.79 2.1 [0.5-6.0]à 82.7è
±63.2* # # 14 PO
(0.15mg/kg/day)ß24.5± 13.7 1.5 [0.4-4.0]à 142è±116 * # # Due to intersubject variability in tacrolimus pharmacokinetics, individualization of dosing regimen is necessary for optimal therapy. (See DOSAGE AND ADMINISTRATION). Pharmacokinetic data indicate that whole blood concentrations rather than plasma concentrations serve as the more appropriate sampling compartment to describe tacrolimus pharmacokinetics.
Absorption
Absorption of tacrolimus from the gastrointestinal tract after oral administration is incomplete and variable. The absolute bioavailability of tacrolimus was 17±10% in adult kidney transplant patients (N=26), 22±6% in adult liver transplant patients (N=17), 23±9% in adult heart transplant patients (N=11) and 18±5% in healthy volunteers (N=16).
A single dose study conducted in 32 healthy volunteers established the bioequivalence of the 1 mg and 5 mg capsules. Another single dose study in 32 healthy volunteers established the bioequivalence of the 0.5 mg and 1 mg capsules. Tacrolimus maximum blood concentrations (Cmax) and area under the curve (AUC) appeared to increase in a dose-proportional fashion in 18 fasted healthy volunteers receiving a single oral dose of 3, 7, and 10 mg.
In 18 kidney transplant patients, tacrolimus trough concentrations from 3 to 30 ng/mL measured at 10-12 hours post-dose (Cmin) correlated well with the AUC (correlation coefficient 0.93). In 24 liver transplant patients over a concentration range of 10 to 60 ng/mL, the correlation coefficient was 0.94. In 25 heart transplant patients over a concentration range of 2 to 24 ng/mL, the correlation coefficient was 0.89 after an oral dose of 0.075 or 0.15 mg/kg/day at steady-state.
Food Effects
The rate and extent of tacrolimus absorption were greatest under fasted conditions. The presence and composition of food decreased both the rate and extent of tacrolimus absorption when administered to 15 healthy volunteers.
The effect was most pronounced with a high-fat meal (848 kcal, 46% fat): mean AUC and Cmax were decreased 37% and 77%, respectively; Tmax was lengthened 5-fold. A high-carbohydrate meal (668 kcal, 85% carbohydrate) decreased mean AUC and mean Cmax by 28% and 65%, respectively.
In healthy volunteers (N=16), the time of the meal also affected tacrolimus bioavailability. When given immediately following the meal, mean Cmax was reduced 71%, and mean AUC was reduced 39%, relative to the fasted condition. When administered 1.5 hours following the meal, mean Cmax was reduced 63%, and mean AUC was reduced 39%, relative to the fasted condition.
In 11 liver transplant patients, Prograf administered 15 minutes after a high fat (400 kcal, 34% fat) breakfast, resulted in decreased AUC (27±18%) and Cmax (50±19%), as compared to a fasted state.
Distribution
The plasma protein binding of tacrolimus is approximately 99% and is independent of concentration over a range of 5-50 ng/mL. Tacrolimus is bound mainly to albumin and alpha-1-acid glycoprotein, and has a high level of association with erythrocytes. The distribution of tacrolimus between whole blood and plasma depends on several factors, such as hematocrit, temperature at the time of plasma separation, drug concentration, and plasma protein concentration. In a U.S. study, the ratio of whole blood concentration to plasma concentration averaged 35 (range 12 to 67).
Metabolism
Tacrolimus is extensively metabolized by the mixed-function oxidase system, primarily the cytochrome P-450 system (CYP3A). A metabolic pathway leading to the formation of 8 possible metabolites has been proposed. Demethylation and hydroxylation were identified as the primary mechanisms of biotransformation in vitro. The major metabolite identified in incubations with human liver microsomes is 13-demethyl tacrolimus. In in vitro studies, a 31-demethyl metabolite has been reported to have the same activity as tacrolimus.
Excretion
The mean clearance following IV administration of tacrolimus is 0.040, 0.083, and 0.053, and 0.051 L/hr/kg in healthy volunteers, adult kidney transplant patients, adult liver transplant patients, and adult heart transplant patients, respectively. In man, less than 1% of the dose administered is excreted unchanged in urine.
In a mass balance study of IV administered radiolabeled tacrolimus to 6 healthy volunteers, the mean recovery of radiolabel was 77.8±12.7%. Fecal elimination accounted for 92.4±1.0% and the elimination half-life based on radioactivity was 48.1±15.9 hours whereas it was 43.5±11.6 hours based on tacrolimus concentrations. The mean clearance of radiolabel was 0.029±0.015 L/hr/kg and clearance of tacrolimus was 0.029±0.009 L/hr/kg. When administered PO, the mean recovery of the radiolabel was 94.9±30.7%. Fecal elimination accounted for 92.6±30.7%, urinary elimination accounted for 2.3±1.1% and the elimination half-life based on radioactivity was 31.9±10.5 hours whereas it was 48.4± 12.3 hours based on tacrolimus concentrations. The mean clearance of radiolabel was 0.226±0.116 L/hr/kg and clearance of tacrolimus 0.172± 0.088 L/hr/kg.
Special Populations
Pediatric
Pharmacokinetics of tacrolimus have been studied in liver transplantation patients, 0.7 to 13.2 years of age. Following IV administration of a 0.037 mg/kg/day dose to 12 pediatric patients, mean terminal half-life, volume of distribution and clearance were 11.5±3.8 hours, 2.6±2.1 L/kg and 0.138± 0.071 L/hr/kg, respectively. Following oral administration to 9 patients, mean AUC and Cmax were 337±167 ng·hr/mL and 48.4±27.9 ng/mL, respectively. The absolute bioavailability was 31±24%.
Whole blood trough concentrations from 31 patients less than 12 years old showed that pediatric patients needed higher doses than adults to achieve similar tacrolimus trough concentrations. (See DOSAGE AND ADMINISTRATION).
Renal and Hepatic Insufficiency
The mean pharmacokinetic parameters for tacrolimus following single administrations to patients with renal and hepatic impairment are given in the following table
- * corrected for bioavailability
- † 1 patient did not receive the PO dose
Population
(No. of Patients)Dose AUC0-t
(ng·hr/
mL)t1/2
(hr)V
(L/kg)CI
(L/hr/kg)
Renal
Impairment
(n=12)0.02
mg/kg/4hr
IV393±123
(t=60 hr)26.3 ±9.2 1.07
±0.200.038
±0.014
Mild Hepatic
Impairment
(n=6)0.02
mg/kg/4hr
IV
367±107
(t=72 hr)
60.6±43.8
Range: 27.8 – 141
3.1±1.6
0.042
±0.02
7.7 mg
PO488±320
(t=72 hr)66.1±44.8
Range: 29.5 – 1383.7±4.7* 0.034
±0.019*
Severe
Hepatic
Impairment
(n=6, IV)
0.02 mg/kg/4hr
IV (n=2)
0.01 mg/kg/8hr
IV (n=4)
762±204
(t=120 hr)
289±117
(t=144 hr)
198±158
Range:81-436
3.9±1.0
0.017
±0.013
(n=5, PO)† 8 mg PO
(n=1)
5 mg PO
(n=4)
4 mg PO
(n=1)658
(t=120 hr)
533±156 (t=144 hr)
119±35
Range: 85-1783.1±3.4* 0.016
±0.011*Renal Insufficiency: Tacrolimus pharmacokinetics following a single IV administration were determined in 12 patients (7 not on dialysis and 5 on dialysis, serum creatinine of 3.9±1.6 and 12.0±2.4 mg/dL, respectively) prior to their kidney transplant. The pharmacokinetic parameters obtained were similar for both groups.
The mean clearance of tacrolimus in patients with renal dysfunction was similar to that in normal volunteers (see previous table).
Hepatic Insufficiency: Tacrolimus pharmacokinetics have been determined in six patients with mild hepatic dysfunction (mean Pugh score: 6.2) following single IV and oral administrations. The mean clearance of tacrolimus in patients with mild hepatic dysfunction was not substantially different from that in normal volunteers (see previous table). Tacrolimus pharmacokinetics were studied in 6 patients with severe hepatic dysfunction (mean Pugh score: >10). The mean clearance was substantially lower in patients with severe hepatic dysfunction, irrespective of the route of administration.
Race
A formal study to evaluate the pharmacokinetic disposition of tacrolimus in Black transplant patients has not been conducted. However, a retrospective comparison of Black and Caucasian kidney transplant patients indicated that Black patients required higher tacrolimus doses to attain similar trough concentrations. (See DOSAGE AND ADMINISTRATION.)
Gender
A formal study to evaluate the effect of gender on tacrolimus pharmacokinetics has not been conducted, however, there was no difference in dosing by gender in the kidney transplant trial. A retrospective comparison of pharmacokinetics in healthy volunteers, and in kidney, liver and heart transplant patients indicated no gender-based differences.
-
CLINICAL STUDIES
Liver Transplantation
The safety and efficacy of Prograf-based immunosuppression following orthotopic liver transplantation were assessed in two prospective, randomized, non-blinded multicenter studies. The active control groups were treated with a cyclosporine-based immunosuppressive regimen. Both studies used concomitant adrenal corticosteroids as part of the immunosuppressive regimens. These studies were designed to evaluate whether the two regimens were therapeutically equivalent, with patient and graft survival at 12 months following transplantation as the primary endpoints. The Prograf-based immunosuppressive regimen was found to be equivalent to the cyclosporine-based immunosuppressive regimens.
In one trial, 529 patients were enrolled at 12 clinical sites in the United States; prior to surgery, 263 were randomized to the Prograf-based immunosuppressive regimen and 266 to a cyclosporine-based immunosuppressive regimen (CBIR). In 10 of the 12 sites, the same CBIR protocol was used, while 2 sites used different control protocols. This trial excluded patients with renal dysfunction, fulminant hepatic failure with Stage IV encephalopathy, and cancers; pediatric patients (≤ 12 years old) were allowed.
In the second trial, 545 patients were enrolled at 8 clinical sites in Europe; prior to surgery, 270 were randomized to the Prograf-based immunosuppressive regimen and 275 to CBIR. In this study, each center used its local standard CBIR protocol in the active-control arm. This trial excluded pediatric patients, but did allow enrollment of subjects with renal dysfunction, fulminant hepatic failure in Stage IV encephalopathy, and cancers other than primary hepatic with metastases.
One-year patient survival and graft survival in the Prograf-based treatment groups were equivalent to those in the CBIR treatment groups in both studies. The overall 1-year patient survival (CBIR and Prograf-based treatment groups combined) was 88% in the U.S. study and 78% in the European study. The overall 1-year graft survival (CBIR and Prograf-based treatment groups combined) was 81% in the U.S. study and 73% in the European study. In both studies, the median time to convert from IV to oral Prograf dosing was 2 days.
Because of the nature of the study design, comparisons of differences in secondary endpoints, such as incidence of acute rejection, refractory rejection or use of OKT3 for steroid-resistant rejection, could not be reliably made.
Kidney Transplantation
Prograf/azathioprine
Prograf-based immunosuppression in conjunction with azathioprine and corticosteroids following kidney transplantation was assessed in a Phase 3 randomized, multicenter, non-blinded, prospective study. There were 412 kidney transplant patients enrolled at 19 clinical sites in the United States. Study therapy was initiated when renal function was stable as indicated by a serum creatinine ≤ 4 mg/dL (median of 4 days after transplantation, range 1 to 14 days). Patients less than 6 years of age were excluded.
There were 205 patients randomized to Prograf-based immunosuppression and 207 patients were randomized to cyclosporine-based immunosuppression. All patients received prophylactic induction therapy consisting of an antilymphocyte antibody preparation, corticosteroids and azathioprine. Overall 1 year patient and graft survival was 96.1% and 89.6%, respectively and was equivalent between treatment arms.
Because of the nature of the study design, comparisons of differences in secondary endpoints, such as incidence of acute rejection, refractory rejection or use of OKT3 for steroid-resistant rejection, could not be reliably made.
Prograf/mycophenolate mofetil (MMF)
Prograf-based immunosuppression in conjunction with MMF, corticosteroids, and induction has been studied. In a randomized, open-label, multi-center trial (Study 1), 1589 kidney transplant patients received Prograf (Group C, n=401), sirolimus (Group D, n=399), or one of two cyclosporine regimens (Group A, n=390 and Group B, n=399) in combination with MMF and corticosteroids; all patients, except those in one of the two cyclosporine groups, also received induction with daclizumab. The study was conducted outside the United States; the study population was 93% Caucasian. In this study, mortality at 12 months in patients receiving Prograf/MMF was similar (2.7%) compared to patients receiving cyclosporine/MMF (3.3% and 1.8%) or sirolimus/MMF (3.0%). Patients in the Prograf group exhibited higher estimated creatinine clearance rates (eCLcr) using the Cockcroft-Gault formula (Table 1) and experienced fewer efficacy failures, defined as biopsy proven acute rejection (BPAR), graft loss, death, and/or lost to follow-up (Table 2) in comparison to each of the other three groups. Patients randomized to Prograf/MMF were more likely to develop diarrhea and diabetes after the transplantation and experienced similar rates of infections compared to patients randomized to either cyclosporine/MMF regimen (see ADVERSE REACTIONS).
Table 1: Estimated Creatinine Clearance at 12 Months in Study 1 Key: CsA=Cyclosporine, CS=Corticosteroids, Tac=Tacrolimus, Siro=Sirolimus - * All death/graft loss (n=41, 27, 23 and 42 in Groups A, B, C and D) and patients whose last recorded creatinine values were prior to month 3 visit (n=10, 9, 7 and 9 in Groups A, B, C and D) were inputed with GFR of 10 mL/min; a subject's last observed creatinine value from month 3 on was used for the remainder of subjects with missing creatinine at month 12 (n=11, 12, 15 and 19 for Groups A, B, C and D). Weight was also imputed in the calculation of estimated GFR, if missing.
- † Adjusted for multiple (6) pairwise comparisons using Bonferroni corrections.
Group eCLcr [mL/min] at Month 12 * N MEAN SD MEDIAN
Treatment Difference with Group C (99.2% CI †) (A) CsA/MMF/CS 390 56.5 25.8 56.9 -8.6 (-13.7, -3.7) (B) CsA/MMF/CS/Daclizumab 399 58.9 25.6 60.9 -6.2 (-11.2, -1.2) (C) Tac/MMF/CS/Daclizumab 401 65.1 27.4 66.2 - (D) Siro/MMF/CS/Daclizumab 399 56.2 27.4 57.3 -8.9 (-14.1, -3.9) Total 1589 59.2 26.8 60.5 Table 2: Incidence of BPAR, Graft Loss, Death or Loss to Follow-up at 12 Months in Study 1 Group A =CsA/MMF/CS, B =CsA/MMF/CS/Daclizumab, C=Tac/MMF/CS/Daclizumab, and D=Siro/MMF/CS/Daclizumab - * Adjusted for multiple (6) pairwise comparisons using Bonferroni corrections.
A
N=390B
N=399C
N=401D
N=399Overall Failure
Components of efficacy failure
BPAR
Graft loss excluding death
Mortality
Lost to follow-up
Treatment Difference of efficacy failure compared to Group C (99.2% CI *)141 (36.2%)
113 (29.0%)
28 (7.2%)
13 (3.3%)
5 (1.3%)
15.8% (7.1%, 24.3%)126 (31.6%)
106 (26.6%)
20 (5.0%)
7 (1.8%)
7 (1.8%)
11.2% (2.7%, 19.5%)82 (20.4%)
60 (15.0%)
12 (3.0%)
11 (2.7%)
5 (1.3%)
-185 (46.4%)
152 (38.1%)
30 (7.5%)
12 (3.0%)
6 (1.5%)
26.0% (17.2%, 34.7%)The protocol-specified target tacrolimus trough concentrations (Ctrough,Tac) were 3-7 ng/mL; however, the observed median Ctroughs,Tac approximated 7 ng/mL throughout the 12 month study (Table 3).
Table 3: Tacrolimus Whole Blood Trough Concentrations (Study 1) - * Range of Ctrough, Tac that excludes lowest 10% and highest 10% of Ctrough, Tac
Time Median (P10-P90 *) tacrolimus whole blood trough concentrations
(ng/mL)Day 30 (N=366) 6.9 (4.4 – 11.3) Day 90 (N=351) 6.8 (4.1 – 10.7) Day 180(N=355) 6.5 (4.0 – 9.6) Day 365 (N=346) 6.5 (3.8 – 10.0) The protocol-specified target cyclosporine trough concentrations (Ctrough,CsA) for Group B were 50-100 ng/mL; however, the observed median Ctroughs,CsA approximated 100 ng/mL throughout the 12 month study. The protocol-specified target Ctroughs,CsA for Group A were 150-300 ng/mL for the first 3 months and 100-200 ng/mL from month 4 to month 12; the observed median Ctroughs, CsA approximated 225 ng/mL for the first 3 months and 140 ng/mL from month 4 to month 12.
While patients in all groups started MMF at 1g BID, the MMF dose was reduced to <2 g/day in 63% of patients in the tacrolimus treatment arm by month 12 (Table 4); approximately 50% of these MMF dose reductions were due to adverse events. By comparison, the MMF dose was reduced to <2 g/day in 49% and 45% of patients in the two cyclosporine arms (Group A and Group B, respectively), by month 12 and approximately 40% of MMF dose reductions were due to adverse events.
Table 4: MMF Dose Over Time in Prograf/MMF (Group C) (Study 1) Time-averaged MMF dose = (total MMF dose)/(duration of treatment) - * Percentage of patients for each time-averaged MMF dose range during various treatment periods. Two g/day of time-averaged MMF dose means that MMF dose was not reduced in those patients during the treatment periods.
Time period (Days) Time-averaged MMF dose (g/day) * <2.0 2.0 >2.0 0-30 (N=364) 37% 60% 2% 0-90 (N=373) 47% 51% 2% 0-180 (N=377) 56% 42% 2% 0-365 (N=380) 63% 36% 1% In a second randomized, open-label, multi-center trial (Study 2), 424 kidney transplant patients received Prograf (n=212) or cyclosporine (n=212) in combination with MMF 1 gram BID, basiliximab induction, and corticosteroids. In this study, the rate for the combined endpoint of biopsy proven acute rejection, graft failure, death, and/or lost to follow-up at 12 months in the Prograf/MMF group was similar to the rate in the cyclosporine/MMF group. There was, however, an imbalance in mortality at 12 months in those patients receiving Prograf/MMF (4.2%) compared to those receiving cyclosporine/MMF (2.4%), including cases attributed to overimmunosuppression (Table 5).
Table 5: Incidence of BPAR, Graft Loss, Death or Loss to Follow-up at 12 Months in Study 2 - * 95% confidence interval calculated using Fisher's Exact Test
Prograf/MMF Cyclosporine/MMF (n=212) (n=212) Overall Failure 32 (15.1%) 36 (17.0%) Components of efficacy failure BPAR 16 (7.5%) 29 (13.7%) Graft loss excluding death 6 (2.8%) 4 (1.9%) Mortality 9 (4.2%) 5 (2.4%) Lost to follow-up 4 (1.9%) 1 (0.5%) Treatment Difference of efficacy failure compared to Prograf/MMF group (95% CI*) - 1.9% (-5.2%, 9.0%) The protocol-specified target tacrolimus whole blood trough concentrations (Ctrough,Tac) in Study 2 were 7-16 ng/mL for the first three months and 5-15 ng/mL thereafter. The observed median Ctroughs,Tac approximated 10 ng/mL during the first three months and 8 ng/mL from month 4 to month 12 (Table 6).
Table 6: Tacrolimus Whole Blood Trough Concentrations (Study 2) - * Range of Ctrough,Tac that excludes lowest 10% and highest 10% of Ctrough, Tac
Time Median (P10-P90*) tacrolimus whole blood trough concentrations
(ng/mL)Day 30 (N=174) 10.5 (6.3 – 16.8) Day 60 (N=179) 9.2 (5.9 – 15.3) Day 120 (N=176) 8.3 (4.6 – 13.3) Day 180 (N=171) 7.8 (5.5 – 13.2) Day 365 (N=178) 7.1 (4.2 – 12.4) The protocol-specified target cyclosporine whole blood concentrations (Ctrough,CsA) were 125 to 400 ng/mL for the first three months, and 100 to 300 ng/mL thereafter. The observed median Ctroughs, CsA approximated 280 ng/mL during the first three months and 190 ng/mL from month 4 to month 12.
Patients in both groups started MMF at 1g BID. The MMF dose was reduced to <2 g/day by month 12 in 62% of patients in the Prograf/MMF group (Table 7) and in 47% of patients in the cyclosporine/MMF group. Approximately 63% and 55% of these MMF dose reductions were because of adverse events in the Prograf/MMF group and the cyclosporine/MMF group, respectively.
Table 7: MMF Dose Over Time in the Prograf/MMF group (Study 2) Time-averaged MMF dose=(total MMF dose)/(duration of treatment) - * Percentage of patients for each time-averaged MMF dose range during various treatment periods. Two g/day of time-averaged MMF dose means that MMF dose was not reduced in those patients during the treatment periods.
Time period (Days) Time-averaged MMF dose (g/day) * <2.0 2.0 >2.0 0-30 (N=212) 25% 69% 6% 0-90 (N=212) 41% 53% 6% 0-180 (N=212) 52% 41% 7% 0-365 (N=212) 62% 34% 4% Heart Transplantation
Two open-label, randomized, comparative studies evaluated the safety and efficacy of Prograf-based and cyclosporine-based immunosuppression in primary orthotopic heart transplantation. In a Phase 3 study conducted in Europe, 314 patients received a regimen of antibody induction, corticosteroids and azathioprine in combination with Prograf or cyclosporine modified for 18 months. In a 3-arm study conducted in the US, 331 patients received corticosteroids and Prograf plus sirolimus, Prograf plus mycophenolate mofetil (MMF) or cyclosporine modified plus MMF for 1 year.
In the European Phase 3 study, patient/graft survival at 18 months posttransplant was similar between treatment arms, 91.7% in the tacrolimus group and 89.2% in the cyclosporine group. In the US study, patient and graft survival at 12 months was similar with 93.5% survival in the Prograf plus MMF group and 86.1% survival in the cyclosporine modified plus MMF group. In the European study, the cyclosporine trough concentrations were above the pre-defined target range (i.e., 100-200 ng/mL) at Day 122 and beyond in 32-68% of the patients in the cyclosporine treatment arm, whereas the tacrolimus trough concentrations were within the pre-defined target range (i.e., 5-15 ng/mL) in 74-86% of the patients in the tacrolimus treatment arm.
The US study contained a third arm of a combination regimen of sirolimus, 2 mg per day, and full-dose Prograf; however, this regimen was associated with increased risk of wound healing complications, renal function impairment, and insulin-dependent post-transplant diabetes mellitus, and is not recommended (see WARNINGS).
-
INDICATIONS AND USAGE
Prograf is indicated for the prophylaxis of organ rejection in patients receiving allogeneic liver, kidney, or heart transplants. It is recommended that Prograf be used concomitantly with adrenal corticosteroids. Because of the risk of anaphylaxis, Prograf injection should be reserved for patients unable to take Prograf capsules orally. In heart and kidney transplant recipients, it is recommended that Prograf be used in conjunction with azathioprine or mycophenolate mofetil (MMF). The safety and efficacy of the use of Prograf with sirolimus has not been established (see CLINICAL STUDIES).
- CONTRAINDICATIONS
-
WARNINGS
(See boxed WARNING)
Post-Transplant Diabetes Mellitus
Insulin-dependent post-transplant diabetes mellitus (PTDM) was reported in 20% of Prograf-treated kidney transplant patients without pretransplant history of diabetes mellitus in the Phase III study (See Tables Below). The median time to onset of PTDM was 68 days. Insulin dependence was reversible in 15% of these PTDM patients at one year and in 50% at 2 years post transplant. Black and Hispanic kidney transplant patients were at an increased risk of development of PTDM.
Incidence of Post Transplant Diabetes Mellitus and Insulin Use at 2 Years in Kidney Transplant Recipients in the Phase III study - * use of insulin for 30 or more consecutive days, with < 5 day gap, without a prior history of insulin dependent diabetes mellitus or non insulin dependent diabetes mellitus.
Status of PTDM* Prograf CBIR
Patients without pretransplant history of diabetes mellitus. 151 151
New onset PTDM*, 1st Year 30/151 (20%) 6/151 (4%)
Still insulin dependent at one year in those without prior history of diabetes. 25/151 (17%) 5/151 (3%)
New onset PTDM* post 1 year 1 0
Patients with PTDM* at 2 years 16/151 (11%) 5/151 (3%) Development of Post Transplant Diabetes Mellitus by Race and by Treatment Group during First Year Post Kidney Transplantation in the Phase III study - * use of insulin for 30 or more consecutive days, with < 5 day gap, without a prior history of insulin dependent diabetes mellitus or non insulin dependent diabetes mellitus.
Patient
RacePrograf CBIR No. of Patients at Risk Patients Who Developed PTDM* No. of Patients At Risk Patients Who Developed PTDM*
Black 41 15 (37%) 36 3 (8%)
Hispanic 17 5 (29%) 18 1 (6%)
Caucasian 82 10 (12%) 87 1 (1%)
Other 11 0 (0%) 10 1 (10%)
Total 151 30 (20%) 151 6 (4%) Insulin-dependent post-transplant diabetes mellitus was reported in 18% and 11% of Prograf-treated liver transplant patients and was reversible in 45% and 31% of these patients at 1 year post transplant, in the U.S. and European randomized studies, respectively (See Table below). Hyperglycemia was associated with the use of Prograf in 47% and 33% of liver transplant recipients in the U.S. and European randomized studies, respectively, and may require treatment (see ADVERSE REACTIONS).
Incidence of Post Transplant Diabetes Mellitus and Insulin Use at 1 Year in Liver Transplant Recipients - * use of insulin for 30 or more consecutive days, with < 5 day gap, without a prior history of insulin dependent diabetes mellitus or non insulin dependent diabetes mellitus.
- † Patients without pretransplant history of diabetes mellitus.
Status of PTDM* US Study European Study Prograf CBIR Prograf CBIR
Patients at risk† 239 236 239 249
New Onset PTDM* 42 (18%) 30 (13%) 26 (11%) 12 (5%)
Patients still on insulin at 1 year 23 (10%) 19 (8%) 18 (8%) 6 (2%) Insulin-dependent post-transplant diabetes mellitus was reported in 13% and 22% of Prograf-treated heart transplant patients receiving mycophenolate mofetil or azathioprine and was reversible in 30% and 17% of these patients at one year post transplant, in the US and European randomized studies, respectively (See Table below). Hyperglycemia defined as two fasting plasma glucose levels ≥126 mg/dL was reported with the use of Prograf plus mycophenolate mofetil or azathioprine in 32% and 35% of heart transplant recipients in the US and European randomized studies, respectively, and may require treatment (see ADVERSE REACTIONS).
Incidence of Post Transplant Diabetes Mellitus and Insulin Use at 1 Year in Heart Transplant Recipients - * use of insulin for 30 or more consecutive days without a prior history of insulin dependent diabetes mellitus or non insulin dependent diabetes mellitus.
- † Patients without pretransplant history of diabetes mellitus.
- ‡ 7-12 months for the US Study.
Status of PTDM* US Study European Study Prograf/Sirolimus Prograf/MMF
Cyclosporine/MMF Prograf/AZA Cyclosporine/AZA
Patients at risk† 85 75 83 132 138
New Onset PTDM* 21 (25%) 10 (13%) 6 (7%) 29 (22%) 5 (4%)
Patients still on insulin at 1 year‡ 10 (12%) 7 (9%) 1 (1%) 24 (18%) 4 (3%) Nephrotoxicity
Prograf can cause nephrotoxicity, particularly when used in high doses. Nephrotoxicity was reported in approximately 52% of kidney transplantation patients and in 40% and 36% of liver transplantation patients receiving Prograf in the U.S. and European randomized trials, respectively, and in 59% of heart transplantation patients in a European randomized trial (see ADVERSE REACTIONS). Use of Prograf with sirolimus in heart transplantation patients in a US study was associated with increased risk of renal function impairment, and is not recommended (See CLINICAL STUDIES). More overt nephrotoxicity is seen early after transplantation, characterized by increasing serum creatinine and a decrease in urine output. Patients with impaired renal function should be monitored closely as the dosage of Prograf may need to be reduced. In patients with persistent elevations of serum creatinine who are unresponsive to dosage adjustments, consideration should be given to changing to another immunosuppressive therapy. Care should be taken in using tacrolimus with other nephrotoxic drugs. In particular, to avoid excess nephrotoxicity, Prograf should not be used simultaneously with cyclosporine. Prograf or cyclosporine should be discontinued at least 24 hours prior to initiating the other. In the presence of elevated Prograf or cyclosporine concentrations, dosing with the other drug usually should be further delayed.
Hyperkalemia
Mild to severe hyperkalemia was reported in 31% of kidney transplant recipients and in 45% and 13% of liver transplant recipients treated with Prograf in the U.S. and European randomized trials, respectively, and in 8% of heart transplant recipients in a European randomized trial and may require treatment (see ADVERSE REACTIONS). Serum potassium levels should be monitored and potassium-sparing diuretics should not be used during Prograf therapy (see PRECAUTIONS).
Neurotoxicity
Prograf can cause neurotoxicity, particularly when used in high doses. Neurotoxicity, including tremor, headache, and other changes in motor function, mental status, and sensory function were reported in approximately 55% of liver transplant recipients in the two randomized studies. Tremor occurred more often in Prograf-treated kidney transplant patients (54%) and heart transplant patients (15%) compared to cyclosporine-treated patients. The incidence of other neurological events in kidney transplant and heart transplant patients was similar in the two treatment groups (see ADVERSE REACTIONS). Tremor and headache have been associated with high whole-blood concentrations of tacrolimus and may respond to dosage adjustment. Seizures have occurred in adult and pediatric patients receiving Prograf (see ADVERSE REACTIONS). Coma and delirium also have been associated with high plasma concentrations of tacrolimus.
Patients treated with tacrolimus have been reported to develop posterior reversible encephalopathy syndrome (PRES). Symptoms indicating PRES include headache, altered mental status, seizures, visual disturbances and hypertension. Diagnosis may be confirmed by radiological procedure. If PRES is suspected or diagnosed, blood pressure control should be maintained and immediate reduction of immunosuppression is advised. This syndrome is characterized by reversal of symptoms upon reduction or discontinuation of immunosuppression.
Malignancy and Lymphoproliferative Disorders
As in patients receiving other immunosuppressants, patients receiving Prograf are at increased risk of developing lymphomas and other malignancies, particularly of the skin. The risk appears to be related to the intensity and duration of immunosuppression rather than to the use of any specific agent. A lymphoproliferative disorder (LPD) related to Epstein-Barr Virus (EBV) infection has been reported in immunosuppressed organ transplant recipients. The risk of LPD appears greatest in young children who are at risk for primary EBV infection while immunosuppressed or who are switched to Prograf following long-term immunosuppression therapy. Because of the danger of oversuppression of the immune system which can increase susceptibility to infection, combination immunosuppressant therapy should be used with caution.
Latent Viral Infections
Immunosuppressed patients are at increased risk for opportunistic infections, including activation of latent viral infections. These include BK virus associated nephropathy and JC virus associated progressive multifocal leukoencephalopathy (PML) which have been observed in patients receiving tacrolimus. These infections may lead to serious, including fatal, outcomes.
Prograf in Combination with Sirolimus
The use of full-dose Prograf with sirolimus (2 mg per day) in heart transplant recipients was associated with increased risk of wound healing complications, renal function impairment, and insulin-dependent post-transplant diabetes mellitus, and is not recommended (see CLINICAL STUDIES).
Anaphylactic Reactions
A few patients receiving Prograf injection have experienced anaphylactic reactions. Although the exact cause of these reactions is not known, other drugs with castor oil derivatives in the formulation have been associated with anaphylaxis in a small percentage of patients. Because of this potential risk of anaphylaxis, Prograf injection should be reserved for patients who are unable to take Prograf capsules.
Patients receiving Prograf injection should be under continuous observation for at least the first 30 minutes following the start of the infusion and at frequent intervals thereafter. If signs or symptoms of anaphylaxis occur, the infusion should be stopped. An aqueous solution of epinephrine should be available at the bedside as well as a source of oxygen.
-
PRECAUTIONS
General
Hypertension is a common adverse effect of Prograf therapy (see ADVERSE REACTIONS). Mild or moderate hypertension is more frequently reported than severe hypertension. Antihypertensive therapy may be required; the control of blood pressure can be accomplished with any of the common antihypertensive agents. Since tacrolimus may cause hyperkalemia, potassium-sparing diuretics should be avoided. While calcium-channel blocking agents can be effective in treating Prograf-associated hypertension, care should be taken since interference with tacrolimus metabolism may require a dosage reduction (see Drug Interactions).
Renally and Hepatically Impaired Patients
For patients with renal insufficiency some evidence suggests that lower doses should be used (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
The use of Prograf in liver transplant recipients experiencing post-transplant hepatic impairment may be associated with increased risk of developing renal insufficiency related to high whole-blood levels of tacrolimus. These patients should be monitored closely and dosage adjustments should be considered. Some evidence suggests that lower doses should be used in these patients (see DOSAGE AND ADMINISTRATION).
Myocardial Hypertrophy
Myocardial hypertrophy has been reported in association with the administration of Prograf, and is generally manifested by echocardiographically demonstrated concentric increases in left ventricular posterior wall and interventricular septum thickness. Hypertrophy has been observed in infants, children and adults. This condition appears reversible in most cases following dose reduction or discontinuance of therapy. In a group of 20 patients with pre- and post-treatment echocardiograms who showed evidence of myocardial hypertrophy, mean tacrolimus whole blood concentrations during the period prior to diagnosis of myocardial hypertrophy ranged from 11 to 53 ng/mL in infants (N=10, age 0.4 to 2 years), 4 to 46 ng/mL in children (N=7, age 2 to 15 years) and 11 to 24 ng/mL in adults (N=3, age 37 to 53 years).
In patients who develop renal failure or clinical manifestations of ventricular dysfunction while receiving Prograf therapy, echocardiographic evaluation should be considered. If myocardial hypertrophy is diagnosed, dosage reduction or discontinuation of Prograf should be considered.
Information for Patients
Patients should be informed of the need for repeated appropriate laboratory tests while they are receiving Prograf. They should be given complete dosage instructions, advised of the potential risks during pregnancy, and informed of the increased risk of neoplasia. Patients should be informed that changes in dosage should not be undertaken without first consulting their physician.
Patients should be informed that Prograf can cause diabetes mellitus and should be advised of the need to see their physician if they develop frequent urination, increased thirst or hunger.
As with other immunosuppressive agents, owing to the potential risk of malignant skin changes, exposure to sunlight and ultraviolet (UV) light should be limited by wearing protective clothing and using a sunscreen with a high protection factor.
Laboratory Tests
Serum creatinine, potassium, and fasting glucose should be assessed regularly. Routine monitoring of metabolic and hematologic systems should be performed as clinically warranted.
Drug Interactions
Due to the potential for additive or synergistic impairment of renal function, care should be taken when administering Prograf with drugs that may be associated with renal dysfunction. These include, but are not limited to, aminoglycosides, amphotericin B, and cisplatin. Initial clinical experience with the co-administration of Prograf and cyclosporine resulted in additive/synergistic nephrotoxicity. Patients switched from cyclosporine to Prograf should receive the first Prograf dose no sooner than 24 hours after the last cyclosporine dose. Dosing may be further delayed in the presence of elevated cyclosporine levels.
Drugs that May Alter Tacrolimus Concentrations
Since tacrolimus is metabolized mainly by the CYP3A enzyme systems, substances known to inhibit these enzymes may decrease the metabolism or increase bioavailability of tacrolimus as indicated by increased whole blood or plasma concentrations. Drugs known to induce these enzyme systems may result in an increased metabolism of tacrolimus or decreased bioavailability as indicated by decreased whole blood or plasma concentrations. Monitoring of blood concentrations and appropriate dosage adjustments are essential when such drugs are used concomitantly.
*Drugs That May Increase Tacrolimus Blood Concentrations - * This table is not all inclusive.
- † In a study of 6 normal volunteers, a significant increase in tacrolimus oral bioavailability (14±5% vs. 30±8%) was observed with concomitant ketoconazole administration (200 mg). The apparent oral clearance of tacrolimus during ketoconazole administration was significantly decreased compared to tacrolimus alone (0.430±0.129 L/hr/kg vs. 0.148±0.043 L/hr/kg). Overall, IV clearance of tacrolimus was not significantly changed by ketoconazole co-administration, although it was highly variable between patients.
- ‡ Lansoprazole (CYP2C19, CYP3A4 substrate) may potentially inhibit CYP3A4-mediated metabolism of tacrolimus and thereby substantially increase tacrolimus whole blood concentrations, especially in transplant patients who are intermediate or poor CYP2C19 metabolizers, as compared to those patients who are efficient CYP2C19 metabolizers.
Calcium
Channel BlockersAntifungal
AgentsMacrolide
Antibioticsdiltiazem clotrimazole clarithromycin nicardipine fluconazole erythromycin nifedipine itraconazole troleandomycin verapamil ketoconazole† voriconazole Gastrointestinal
Prokinetic AgentsOther
Drugscisapride bromocriptine metoclopramide chloramphenicol cimetidine cyclosporine danazol ethinyl estradiol methylprednisolone lansoprazole‡ omeprazole protease inhibitors nefazodone magnesium-aluminum-hydroxide *Drugs That May Decrease Tacrolimus Blood Concentrations - * This table is not all inclusive.
Anticonvulsants Antimicrobials carbamazepine rifabutin phenobarbital caspofungin phenytoin rifampin Herbal Preparations Other Drugs St. John’s Wort sirolimus St. John’s Wort (Hypericum perforatum) induces CYP3A4 and P-glycoprotein. Since tacrolimus is a substrate for CYP3A4, there is the potential that the use of St. John’s Wort in patients receiving Prograf could result in reduced tacrolimus levels.
In a single-dose crossover study in healthy volunteers, co-administration of tacrolimus and magnesium-aluminum-hydroxide resulted in a 21% increase in the mean tacrolimus AUC and a 10% decrease in the mean tacrolimus Cmax relative to tacrolimus administration alone.
In a study of 6 normal volunteers, a significant decrease in tacrolimus oral bioavailability (14±6% vs. 7±3%) was observed with concomitant rifampin administration (600 mg). In addition, there was a significant increase in tacrolimus clearance (0.036±0.008 L/hr/kg vs. 0.053±0.010 L/hr/kg) with concomitant rifampin administration.
Interaction studies with drugs used in HIV therapy have not been conducted. However, care should be exercised when drugs that are nephrotoxic (e.g., ganciclovir) or that are metabolized by CYP3A (e.g., nelfinavir, ritonavir) are administered concomitantly with tacrolimus. Based on a clinical study of 5 liver transplant recipients, co-administration of tacrolimus with nelfinavir increased blood concentrations of tacrolimus significantly and, as a result, a reduction in the tacrolimus dose by an average of 16-fold was needed to maintain mean trough tacrolimus blood concentrations of 9.7 ng/mL. Thus, frequent monitoring of tacrolimus blood concentrations and appropriate dosage adjustments are essential when nelfinavir is used concomitantly. Tacrolimus may affect the pharmacokinetics of other drugs (e.g., phenytoin) and increase their concentration. Grapefruit juice affects CYP3A-mediated metabolism and should be avoided (see DOSAGE AND ADMINISTRATION).
Following co-administration of tacrolimus and sirolimus (2 or 5 mg/day) in stable renal transplant patients, mean tacrolimus AUC0-12 and Cmin decreased approximately by 30% relative to tacrolimus alone. Mean tacrolimus AUC0-12 and Cmin following co-administration of 1 mg/day of sirolimus decreased approximately 3% and 11%, respectively. The safety and efficacy of tacrolimus used in combination with sirolimus for the prevention of graft rejection has not been established and is not recommended.
Other Drug Interactions
Immunosuppressants may affect vaccination. Therefore, during treatment with Prograf, vaccination may be less effective. The use of live vaccines should be avoided; live vaccines may include, but are not limited to measles, mumps, rubella, oral polio, BCG, yellow fever, and TY 21a typhoid.1
At a given MMF dose, mycophenolic acid (MPA) exposure is higher with Prograf co-administration than with cyclosporine co-administration due to the differences in the interruption of the enterohepatic recirculation of MPA. Clinicians should be aware that there is also a potential for increased MPA exposure after crossover from cyclosporine to tacrolimus in patients concomitantly receiving MMF or MPA.
Carcinogenesis, Mutagenesis and Impairment of Fertility
An increased incidence of malignancy is a recognized complication of immunosuppression in recipients of organ transplants. The most common forms of neoplasms are non-Hodgkin’s lymphomas and carcinomas of the skin. As with other immunosuppressive therapies, the risk of malignancies in Prograf recipients may be higher than in the normal, healthy population. Lymphoproliferative disorders associated with Epstein-Barr Virus infection have been seen. It has been reported that reduction or discontinuation of immunosuppression may cause the lesions to regress.
No evidence of genotoxicity was seen in bacterial (Salmonella and E. coli) or mammalian (Chinese hamster lung-derived cells) in vitro assays of mutagenicity, the in vitro CHO/HGPRT assay of mutagenicity, or in vivo clastogenicity assays performed in mice; tacrolimus did not cause unscheduled DNA synthesis in rodent hepatocytes.
Carcinogenicity studies were carried out in male and female rats and mice. In the 80-week mouse study and in the 104-week rat study no relationship of tumor incidence to tacrolimus dosage was found. The highest doses used in the mouse and rat studies were 0.8 – 2.5 times (mice) and 3.5 – 7.1 times (rats) the recommended clinical dose range of 0.1 – 0.2 mg/kg/day when corrected for body surface area.
No impairment of fertility was demonstrated in studies of male and female rats. Tacrolimus, given orally at 1.0 mg/kg (0.7 – 1.4X the recommended clinical dose range of 0.1 – 0.2 mg/kg/day based on body surface area corrections) to male and female rats, prior to and during mating, as well as to dams during gestation and lactation, was associated with embryolethality and with adverse effects on female reproduction. Effects on female reproductive function (parturition) and embryolethal effects were indicated by a higher rate of pre-implantation loss and increased numbers of undelivered and nonviable pups. When given at 3.2 mg/kg (2.3 – 4.6X the recommended clinical dose range based on body surface area correction), tacrolimus was associated with maternal and paternal toxicity as well as reproductive toxicity including marked adverse effects on estrus cycles, parturition, pup viability, and pup malformations.
Pregnancy: Category C
In reproduction studies in rats and rabbits, adverse effects on the fetus were observed mainly at dose levels that were toxic to dams. Tacrolimus at oral doses of 0.32 and 1.0 mg/kg during organogenesis in rabbits was associated with maternal toxicity as well as an increase in incidence of abortions; these doses are equivalent to 0.5 – 1X and 1.6 – 3.3X the recommended clinical dose range (0.1 – 0.2 mg/kg) based on body surface area corrections. At the higher dose only, an increased incidence of malformations and developmental variations was also seen. Tacrolimus, at oral doses of 3.2 mg/kg during organogenesis in rats, was associated with maternal toxicity and caused an increase in late resorptions, decreased numbers of live births, and decreased pup weight and viability. Tacrolimus, given orally at 1.0 and 3.2 mg/kg (equivalent to 0.7 – 1.4X and 2.3 – 4.6X the recommended clinical dose range based on body surface area corrections) to pregnant rats after organogenesis and during lactation, was associated with reduced pup weights.
No reduction in male or female fertility was evident.
There are no adequate and well-controlled studies in pregnant women. Tacrolimus is transferred across the placenta. The use of tacrolimus during pregnancy has been associated with neonatal hyperkalemia and renal dysfunction. Prograf should be used during pregnancy only if the potential benefit to the mother justifies potential risk to the fetus.
Pediatric Patients
Experience with Prograf in pediatric kidney and heart transplant patients is limited. Successful liver transplants have been performed in pediatric patients (ages up to 16 years) using Prograf. Two randomized active-controlled trials of Prograf in primary liver transplantation included 56 pediatric patients. Thirty-one patients were randomized to Prograf-based and 25 to cyclosporine-based therapies. Additionally, a minimum of 122 pediatric patients were studied in an uncontrolled trial of tacrolimus in living related donor liver transplantation. Pediatric patients generally required higher doses of Prograf to maintain blood trough concentrations of tacrolimus similar to adult patients (see DOSAGE AND ADMINISTRATION).
-
ADVERSE REACTIONS
Liver Transplantation
The principal adverse reactions of Prograf are tremor, headache, diarrhea, hypertension, nausea, and abnormal renal function. These occur with oral and IV administration of Prograf and may respond to a reduction in dosing. Diarrhea was sometimes associated with other gastrointestinal complaints such as nausea and vomiting.
Hyperkalemia and hypomagnesemia have occurred in patients receiving Prograf therapy. Hyperglycemia has been noted in many patients; some may require insulin therapy (see WARNINGS).
The incidence of adverse events was determined in two randomized comparative liver transplant trials among 514 patients receiving tacrolimus and steroids and 515 patients receiving a cyclosporine-based regimen (CBIR). The proportion of patients reporting more than one adverse event was 99.8% in the tacrolimus group and 99.6% in the CBIR group. Precautions must be taken when comparing the incidence of adverse events in the U.S. study to that in the European study. The 12-month posttransplant information from the U.S. study and from the European study is presented below. The two studies also included different patient populations and patients were treated with immunosuppressive regimens of differing intensities. Adverse events reported in ≥ 15% in tacrolimus patients (combined study results) are presented below for the two controlled trials in liver transplantation:
LIVER TRANSPLANTATION: ADVERSE EVENTS OCCURRING IN ≥ 15% OF PROGRAF-TREATED PATIENTS U.S. STUDY EUROPEAN STUDY Prograf
(N=250)CBIR
(N=250)Prograf
(N=264)CBIR
(N=265)Nervous System
Headache (see WARNINGS)
Tremor (see WARNINGS)
Insomnia
Paresthesia
64%
56%
64%
40%
60%
46%
68%
30%
37%
48%
32%
17%
26%
32%
23%
17%
Gastrointestinal
Diarrhea
Nausea
Constipation
LFT Abnormal
Anorexia
Vomiting
72%
46%
24%
36%
34%
27%
47%
37%
27%
30%
24%
15%
37%
32%
23%
6%
7%
14%
27%
27%
21%
5%
5%
11%
Cardiovascular
Hypertension (see PRECAUTIONS)
47%
56%
38%
43%
Urogenital
Kidney Function Abnormal (see WARNINGS)
Creatinine Increased (see WARNINGS)
BUN Increased (see WARNINGS)
Urinary Tract Infection
Oliguria
40%
39%
30%
16%
18%
27%
25%
22%
18%
15%
36%
24%
12%
21%
19%
23%
19%
9%
19%
12%
Metabolic and Nutritional
Hyperkalemia (see WARNINGS)
Hypokalemia
Hyperglycemia (see WARNINGS)
Hypomagnesemia
45%
29%
47%
48%
26%
34%
38%
45%
13%
13%
33%
16%
9%
16%
22%
9%
Hemic and Lymphatic
Anemia
Leukocytosis
Thrombocytopenia
47%
32%
24%
38%
26%
20%
5%
8%
14%
1%
8%
19%
Miscellaneous
Abdominal Pain
Pain
Fever
Asthenia
Back Pain
Ascites
Peripheral Edema
59%
63%
48%
52%
30%
27%
26%
54%
57%
56%
48%
29%
22%
26%
29%
24%
19%
11%
17%
7%
12%
22%
22%
22%
7%
17%
8%
14%
Respiratory System
Pleural Effusion
Atelectasis
Dyspnea
30%
28%
29%
32%
30%
23%
36%
5%
5%
35%
4%
4%
Skin and Appendages
Pruritus
Rash36%
24%20%
19%15%
10%7%
4%
Less frequently observed adverse reactions in both liver transplantation and kidney transplantation patients are described under the subsection Less Frequently Reported Adverse Reactions below.
Kidney Transplantation
The most common adverse reactions reported were infection, tremor, hypertension, abnormal renal function, constipation, diarrhea, headache, abdominal pain and insomnia.
Adverse events that occurred in ≥ 15% of kidney transplant patients treated with Prograf in conjunction with azathioprine are presented below:
KIDNEY TRANSPLANTATION: ADVERSE EVENTS OCCURRING IN ≥ 15% OF PATIENTS TREATED WITH PROGRAF IN CONJUNCTION WITH AZATHIOPRINE Prograf
(N=205)CBIR
(N=207)Nervous System
Tremor (see WARNINGS)
Headache (see WARNINGS)
Insomnia
Paresthesia
Dizziness
54%
44%
32%
23%
19%
34%
38%
30%
16%
16%
Gastrointestinal
Diarrhea
Nausea
Constipation
Vomiting
Dyspepsia
44%
38%
35%
29%
28%
41%
36%
43%
23%
20%
Cardiovascular
Hypertension (see PRECAUTIONS)
Chest pain
50%
19%
52%
13%
Urogenital
Creatinine Increased (see WARNINGS)
Urinary Tract Infection
45%
34%
42%
35%
Metabolic and Nutritional
Hypophosphatemia
Hypomagnesemia
Hyperlipemia
Hyperkalemia (see WARNINGS)
Diabetes Mellitus (see WARNINGS)
Hypokalemia
Hyperglycemia (see WARNINGS)
Edema
49%
34%
31%
31%
24%
22%
22%
18%
53%
17%
38%
32%
9%
25%
16%
19%
Hemic and Lymphatic
Anemia
Leukopenia
30%
15%
24%
17%
Miscellaneous
Infection
Peripheral Edema
Asthenia
Abdominal Pain
Pain
Fever
Back Pain
45%
36%
34%
33%
32%
29%
24%
49%
48%
30%
31%
30%
29%
20%
Respiratory System
Dyspnea
Cough Increased
22%
18%
18%
15%
Musculoskeletal
Arthralgia
25%
24%
Skin
Rash
Pruritus17%
15%12%
7%Adverse events that occurred in ≥ 10% of kidney transplant patients treated with Prograf in conjunction with MMF in Study 1* are presented below:
*Study 1 was conducted entirely outside of the United States. Such studies often report a lower incidence of adverse events in comparison to US studies. KIDNEY TRANSPLANTATION: ADVERSE EVENTS OCCURRING IN ≥ 10% OF PROGRAF-TREATED PATIENTS Prograf (Group C) Cyclosporine (Group A) Cyclosporine (Group B) (N=403) (N=384) (N=408) Anemia 17% 19% 17% Leucopenia 13% 10% 10% Diarrhea 25% 16% 13% Edema peripheral 11% 12% 13% Urinary tract infection 24% 28% 24% Hyperlipidemia 10% 15% 13% Hypertension (see PRECAUTIONS) 13% 14% 12% Adverse events that occurred in ≥15% of kidney transplant patients treated with Prograf in conjunction with MMF in Study 2 are presented below:
KIDNEY TRANSPLANTATION: ADVERSE EVENTS OCCURRING IN ≥ 15% OF PROGRAF-TREATED PATIENTS Prograf Cyclosporine (N=212) (N=212) Gastrointestinal Disorders Diarrhea 44% 26% Nausea 39% 47% Constipation 36% 41% Vomiting 26% 25% Dyspepsia 18% 15% Injury, Poisoning, and Procedural Complications Post Procedural Pain 29% 27% Incision Site Complication 28% 23% Graft Dysfunction 24% 18% Metabolism and Nutrition Disorders Hypomagnesemia 28% 22% Hypophosphatemia 28% 21% Hyperkalemia (see WARNINGS) 26% 19% Hyperglycemia (see WARNINGS) 21% 15% Hyperlipidemia 18% 25% Hypokalemia 16% 18% Nervous System Disorders Tremor 34% 20% Headache 24% 25% Blood and Lymphatic System Disorders Anemia 30% 28% Leukopenia 16% 12% Miscellaneous Edema Peripheral 35% 46% Hypertension (see PRECAUTIONS) 32% 35% Insomnia 30% 21% Urinary Tract Infection 26% 22% Blood creatinine increased 23% 23% Less frequently observed adverse reactions in both liver transplantation and kidney transplantation patients are described under the subsection Less Frequently Reported Adverse Reactions shown below.
Heart Transplantation
The more common adverse reactions in Prograf-treated heart transplant recipients were abnormal renal function , hypertension, diabetes mellitus, CMV infection, tremor, hyperglycemia, leukopenia, infection, and hyperlipemia.
Adverse events in heart transplant patients in the European trial are presented below:
HEART TRANSPLANTATION: ADVERSE EVENTS OCCURRING IN ≥ 15% OF PROGRAF-TREATED PATIENTS COSTART Body System
COSTART TermPrograf+
Azathioprine
(n=157)CsA + Azathioprine
(n=157)Cardiovascular System Hypertension (See PRECAUTIONS) 62% 69% Pericardial effusion 15% 14% Body as a Whole CMV infection 32% 30% Infection 24% 21% Metabolic and Nutritional Disorders Hyperlipemia 18% 27% Diabetes Mellitus (See WARNINGS) 26% 16% Hyperglycemia (See WARNINGS) 23% 17% Hemic and Lymphatic System Leukopenia 48% 39% Anemia 50% 36% Urogenital System Kidney function abnormal (See WARNINGS) 56% 57% Urinary tract infection 16% 12% Respiratory System Bronchitis 17% 18% Nervous System Tremor (See WARNINGS) 15% 6% In the European study, the cyclosporine trough concentrations were above the pre-defined target range (i.e., 100-200 ng/mL) at Day 122 and beyond in 32-68% of the patients in the cyclosporine treatment arm, whereas the tacrolimus trough concentrations were within the pre-defined target range (i.e., 5-15 ng/mL) in 74-86% of the patients in the tacrolimus treatment arm.
Only selected targeted treatment-emergent adverse events were collected in the US heart transplantation study. Those events that were reported at a rate of 15% or greater in patients treated with Prograf and mycophenolate mofetil include the following: any target adverse events (99.1%), hypertension (88.8%), hyperglycemia requiring antihyperglycemic therapy (70.1%) (see WARNINGS), hypertriglyceridemia (65.4%), anemia (hemoglobin <10.0 g/dL) (65.4%), fasting blood glucose >140 mg/dL (on two separate occasions) (60.7%) (see WARNINGS), hypercholesterolemia (57.0%), hyperlipidemia (33.6%), WBCs <3000 cells/mcL (33.6%), serious bacterial infections (29.9%), magnesium <1.2 mEq/L (24.3%), platelet count <75,000 cells/mcL (18.7%), and other opportunistic infections (15.0%).
Other targeted treatment-emergent adverse events in Prograf-treated patients occurred at a rate of less than 15%, and include the following: Cushingoid features, impaired wound healing, hyperkalemia, Candida infection, and CMV infection/syndrome.
Less Frequently Reported Adverse Reactions
The following adverse events were reported in either liver, kidney, and/or heart transplant recipients who were treated with tacrolimus in clinical trials.
Nervous System
(see WARNINGS)
Abnormal dreams, agitation, amnesia, anxiety, confusion, convulsion, crying, depression, dizziness, elevated mood, emotional lability, encephalopathy, haemorrhagic stroke, hallucinations, headache, hypertonia, incoordination, insomnia, monoparesis, myoclonus, nerve compression, nervousness, neuralgia, neuropathy, paresthesia, paralysis flaccid, psychomotor skills impaired, psychosis, quadriparesis, somnolence, thinking abnormal, vertigo, writing impaired
Gastrointestinal
Anorexia, cholangitis, cholestatic jaundice, diarrhea, duodenitis, dyspepsia, dysphagia, esophagitis, flatulence, gastritis, gastroesophagitis, gastrointestinal hemorrhage, GGT increase, GI disorder, GI perforation, hepatitis, hepatitis granulomatous, ileus, increased appetite, jaundice, liver damage, liver function test abnormal, nausea, nausea and vomiting, oesophagitis ulcerative, oral moniliasis, pancreatic pseudocyst, rectal disorder, stomatitis, vomiting
Cardiovascular
Abnormal ECG, angina pectoris, arrhythmia, atrial fibrillation, atrial flutter, bradycardia, cardiac fibrillation, cardiopulmonary failure, cardiovascular disorder, chest pain, congestive heart failure, deep thrombophlebitis, echocardiogram abnormal, electrocardiogram QRS complex abnormal, electrocardiogram ST segment abnormal, heart failure, heart rate decreased, hemorrhage, hypotension, peripheral vascular disorder, phlebitis, postural hypotension, syncope, tachycardia, thrombosis, vasodilatation
Urogenital
(see WARNINGS)
Acute kidney failure, albuminuria, BK nephropathy, bladder spasm, cystitis, dysuria, hematuria, hydronephrosis, kidney failure, kidney tubular necrosis, nocturia, oliguria, pyuria, toxic nephropathy, urge incontinence, urinary frequency, urinary incontinence, urinary retention, vaginitis
Metabolic/Nutritional
Acidosis, alkaline phosphatase increased, alkalosis, ALT (SGPT) increased, AST (SGOT) increased, bicarbonate decreased, bilirubinemia, BUN increased, dehydration, edema, GGT increased, gout, healing abnormal, hypercalcemia, hypercholesterolemia, hyperkalemia, hyperlipemia, hyperphosphatemia, hyperuricemia, hypervolemia, hypocalcemia, hypoglycemia, hypokalemia, hypomagnesemia, hyponatremia, hypophosphatemia, hypoproteinemia, lactic dehydrogenase increase, peripheral edema, weight gain
Hemic/Lymphatic
Coagulation disorder, ecchymosis, haematocrit increased, haemoglobin abnormal, hypochromic anemia, leukocytosis, leukopenia, polycythemia, prothrombin decreased, serum iron decreased, thrombocytopenia
Miscellaneous
Abdomen enlarged, abdominal pain, abscess, accidental injury, allergic reaction, asthenia, back pain, cellulitis, chills, fall, feeling abnormal, fever, flu syndrome, generalized edema, hernia, mobility decreased, pain, peritonitis, photosensitivity reaction, sepsis, temperature intolerance, ulcer
Musculoskeletal
Arthralgia, cramps, generalized spasm, joint disorder, leg cramps, myalgia, myasthenia, osteoporosis
Post Marketing Adverse Events
The following adverse events have been reported from worldwide marketing experience with Prograf. Because these events are reported voluntarily from a population of uncertain size, are associated with concomitant diseases and multiple drug therapies and surgical procedures, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Decisions to include these events in labeling are typically based on one or more of the following factors: (1) seriousness of the event, (2) frequency of the reporting, or (3) strength of causal connection to the drug.
There have been rare spontaneous reports of myocardial hypertrophy associated with clinically manifested ventricular dysfunction in patients receiving Prograf therapy (see PRECAUTIONS- Myocardial Hypertrophy).
Other events include:
Cardiovascular
Atrial fibrillation, atrial flutter, cardiac arrhythmia, cardiac arrest, electrocardiogram T wave abnormal, flushing, myocardial infarction, myocardial ischaemia, pericardial effusion, QT prolongation, Torsade de Pointes, venous thrombosis deep limb, ventricular extrasystoles, ventricular fibrillation
Gastrointestinal
Bile duct stenosis, colitis, enterocolitis, gastroenteritis, gastrooesophageal reflux disease, hepatic cytolysis, hepatic necrosis, hepatotoxicity, impaired gastric emptying, liver fatty, mouth ulceration, pancreatitis haemorrhagic, pancreatitis necrotizing, stomach ulcer, venoocclusive liver disease
Hemic/Lymphatic
Disseminated intravascular coagulation, neutropenia, pancytopenia, thrombocytopenic purpura, thrombotic thrombocytopenic purpura
Miscellaneous
Feeling hot and cold, feeling jittery, hot flushes, multi-organ failure, primary graft dysfunction
Nervous System
Carpal tunnel syndrome, cerebral infarction, hemiparesis, leukoencephalopathy, mental disorder, mutism, posterior reversible encephalopathy syndrome (PRES), progressive multifocal leukoencephalopathy (PML), quadriplegia, speech disorder, syncope
-
OVERDOSAGE
Limited overdosage experience is available. Acute overdosages of up to 30 times the intended dose have been reported. Almost all cases have been asymptomatic and all patients recovered with no sequelae. Occasionally, acute overdosage has been followed by adverse reactions consistent with those listed in the ADVERSE REACTIONS section except in one case where transient urticaria and lethargy were observed. Based on the poor aqueous solubility and extensive erythrocyte and plasma protein binding, it is anticipated that tacrolimus is not dialyzable to any significant extent; there is no experience with charcoal hemoperfusion. The oral use of activated charcoal has been reported in treating acute overdoses, but experience has not been sufficient to warrant recommending its use. General supportive measures and treatment of specific symptoms should be followed in all cases of overdosage.
In acute oral and IV toxicity studies, mortalities were seen at or above the following doses: in adult rats, 52X the recommended human oral dose; in immature rats, 16X the recommended oral dose; and in adult rats, 16X the recommended human IV dose (all based on body surface area corrections).
-
DOSAGE AND ADMINISTRATION
Prograf injection (tacrolimus injection)
For IV Infusion Only
NOTE: Anaphylactic reactions have occurred with injectables containing castor oil derivatives. SeeWARNINGS.
In patients unable to take oral Prograf capsules, therapy may be initiated with Prograf injection. The initial dose of Prograf should be administered no sooner than 6 hours after transplantation. The recommended starting dose of Prograf injection is 0.01 mg/kg/day (heart) or 0.03-0.05 mg/kg/day (liver, kidney) as a continuous IV infusion. Adult patients should receive doses at the lower end of the dosing range. Concomitant adrenal corticosteroid therapy is recommended early post-transplantation. Continuous IV infusion of Prograf injection should be continued only until the patient can tolerate oral administration of Prograf capsules.
Preparation for Administration/Stability
Prograf injection must be diluted with 0.9% Sodium Chloride Injection or 5% Dextrose Injection to a concentration between 0.004 mg/mL and 0.02 mg/mL prior to use. Diluted infusion solution should be stored in glass or polyethylene containers and should be discarded after 24 hours. The diluted infusion solution should not be stored in a PVC container due to decreased stability and the potential for extraction of phthalates. In situations where more dilute solutions are utilized (e.g., pediatric dosing, etc.), PVC-free tubing should likewise be used to minimize the potential for significant drug adsorption onto the tubing. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Due to the chemical instability of tacrolimus in alkaline media, Prograf injection should not be mixed or co-infused with solutions of pH 9 or greater (e.g., ganciclovir or acyclovir).
Prograf capsules (tacrolimus capsules)
Summary of Initial Oral Dosage Recommendations and Observed Whole Blood Trough Concentrations - * Note : two divided doses, q12h
- † In a second smaller study, the initial dose of tacrolimus was 0.15-0.2 mg/kg/day and observed tacrolimus concentrations were 6-16 ng/mL during month 1-3 and 5-12 ng/mL during month 4-12 (see CLINICAL STUDIES).
Patient Population Recommended Initial
Oral Dosage*Observed Whole Blood Trough Concentrations Adult kidney transplant patients
In combination with azathioprine
In combination with MMF/IL-2 receptor
antagonist †
0.2 mg/kg/day
0.1 mg/kg/day
month 1-3: 7-20 ng/mL
month 4-12: 5-15 ng/mL
month 1-12: 4-11 ng/mL
Adult liver transplant patients 0.10-0.15 mg/kg/day month 1-12: 5-20 ng/mL Pediatric liver transplant patients 0.15-0.20 mg/kg/day month 1-12: 5-20 ng/mL Adult heart transplant patients 0.075 mg/kg/day month 1-3: 10-20 ng/mL
month ≥4: 5-15 ng/mLLiver Transplantation
It is recommended that patients initiate oral therapy with Prograf capsules if possible. If IV therapy is necessary, conversion from IV to oral Prograf is recommended as soon as oral therapy can be tolerated. This usually occurs within 2-3 days. The initial dose of Prograf should be administered no sooner than 6 hours after transplantation. In a patient receiving an IV infusion, the first dose of oral therapy should be given 8-12 hours after discontinuing the IV infusion. The recommended starting oral dose of Prograf capsules is 0.10 to 0.15 mg/kg/day administered in two divided daily doses every 12 hours. Co-administered grapefruit juice has been reported to increase tacrolimus blood trough concentrations in liver transplant patients. (See Drugs that May Alter Tacrolimus Concentrations).
Dosing should be titrated based on clinical assessments of rejection and tolerability. Lower Prograf dosages may be sufficient as maintenance therapy. Adjunct therapy with adrenal corticosteroids is recommended early post-transplant.
Dosage and typical tacrolimus whole blood trough concentrations are shown in the table above; blood concentration details are described in Blood Concentration Monitoring: Liver Transplantation below.
Kidney Transplantation
The recommended starting oral dose of Prograf (administered every 12 hours in two divided doses) is 0.2 mg/kg/day when used in combination with azathioprine or 0.1 mg/kg/day when used in combination with MMF and IL-2 receptor antagonist (see CLINICAL STUDIES). The initial dose of Prograf may be administered within 24 hours of transplantation, but should be delayed until renal function has recovered (as indicated for example by a serum creatinine ≤ 4 mg/dL). Black patients may require higher doses to achieve comparable blood concentrations. Dosage and typical tacrolimus whole blood trough concentrations are shown in the table above; blood concentration details are described in Blood Concentration Monitoring: Kidney Transplantation below.
The data in kidney transplant patients indicate that the Black patients required a higher dose to attain comparable trough concentrations compared to Caucasian patients.
Time After Transplant Caucasian
n=114
Black
n=56Dose
(mg/kg)Trough Concentrations
(ng/mL)Dose
(mg/kg)Trough Concentrations (ng/mL) Day 7 0.18 12.0 0.23 10.9 Month 1 0.17 12.8 0.26 12.9 Month 6 0.14 11.8 0.24 11.5 Month 12 0.13 10.1 0.19 11.0 Heart Transplantation
The recommended starting oral dose of Prograf is 0.075 mg/kg/day administered every 12 hours in two divided doses. If possible, initiating oral therapy with Prograf capsules is recommended. If IV therapy is necessary, conversion from IV to oral Prograf is recommended as soon as oral therapy can be tolerated. This usually occurs within 2-3 days. The initial dose of Prograf should be administered no sooner than 6 hours after transplantation. In a patient receiving an IV infusion, the first dose of oral therapy should be given 8-12 hours after discontinuing the IV infusion.
Dosing should be titrated based on clinical assessments of rejection and tolerability. Lower Prograf dosages may be sufficient as maintenance therapy. Adjunct therapy with adrenal corticosteroids is recommended early post transplant.
Dosage and typical tacrolimus whole blood trough concentrations are shown in the table above; blood concentration details are described in Blood Concentration Monitoring: Heart Transplantationbelow.
Pediatric Patients
Pediatric liver transplantation patients without pre-existing renal or hepatic dysfunction have required and tolerated higher doses than adults to achieve similar blood concentrations. Therefore, it is recommended that therapy be initiated in pediatric patients at a starting IV dose of 0.03-0.05 mg/kg/day and a starting oral dose of 0.15-0.20 mg/kg/day. Dose adjustments may be required. Experience in pediatric kidney and heart transplantation patients is limited.
Patients with Hepatic or Renal Dysfunction
Due to the reduced clearance and prolonged half-life, patients with severe hepatic impairment (Pugh ≥ 10) may require lower doses of Prograf. Close monitoring of blood concentrations is warranted.
Due to the potential for nephrotoxicity, patients with renal or hepatic impairment should receive doses at the lowest value of the recommended IV and oral dosing ranges. Further reductions in dose below these ranges may be required. Prograf therapy usually should be delayed up to 48 hours or longer in patients with post-operative oliguria.
Conversion from One Immunosuppressive Regimen to Another
Prograf should not be used simultaneously with cyclosporine. Prograf or cyclosporine should be discontinued at least 24 hours before initiating the other. In the presence of elevated Prograf or cyclosporine concentrations, dosing with the other drug usually should be further delayed.
Blood Concentration Monitoring
Monitoring of tacrolimus blood concentrations in conjunction with other laboratory and clinical parameters is considered an essential aid to patient management for the evaluation of rejection, toxicity, dose adjustments and compliance. Factors influencing frequency of monitoring include but are not limited to hepatic or renal dysfunction, the addition or discontinuation of potentially interacting drugs and the posttransplant time. Blood concentration monitoring is not a replacement for renal and liver function monitoring and tissue biopsies.
Two methods have been used for the assay of tacrolimus, a microparticle enzyme immunoassay (MEIA) and ELISA. Both methods have the same monoclonal antibody for tacrolimus. Comparison of the concentrations in published literature to patient concentrations using the current assays must be made with detailed knowledge of the assay methods and biological matrices employed. Whole blood is the matrix of choice and specimens should be collected into tubes containing ethylene diamine tetraacetic acid (EDTA) anti-coagulant. Heparin anti-coagulation is not recommended because of the tendency to form clots on storage. Samples which are not analyzed immediately should be stored at room temperature or in a refrigerator and assayed within 7 days; if samples are to be kept longer they should be deep frozen at -20° C for up to 12 months.
Liver Transplantation
Although there is a lack of direct correlation between tacrolimus concentrations and drug efficacy, data from Phase II and III studies of liver transplant patients have shown an increasing incidence of adverse events with increasing trough blood concentrations. Most patients are stable when trough whole blood concentrations are maintained between 5 to 20 ng/mL. Long-term post-transplant patients often are maintained at the low end of this target range.
Data from the U.S. clinical trial show that tacrolimus whole blood concentrations, as measured by ELISA, were most variable during the first week post-transplantation. After this early period, the median trough blood concentrations, measured at intervals from the second week to one year post-transplantation, ranged from 9.8 ng/mL to 19.4 ng/mL.
Therapeutic Drug Monitoring, 1995, Volume 17, Number 6 contains a consensus document and several position papers regarding the therapeutic monitoring of tacrolimus from the 1995 International Consensus Conference on Immunosuppressive Drugs. Refer to these manuscripts for further discussions of tacrolimus monitoring.
Kidney Transplantation
Data from a Phase 3 study of Prograf in conjunction with azathioprine indicate that trough concentrations of tacrolimus in whole blood, as measured by IMx® were most variable during the first week of dosing. During the first three months of that trial, 80% of the patients maintained trough concentrations between 7-20 ng/mL, and then between 5-15 ng/mL, through 1 year.
In a separate clinical trial of Prograf in conjunction with MMF and daclizumab, approximately 80% of patients maintained tacrolimus whole blood concentrations between 4-11 ng/mL through 1 year post-transplant.
In another clinical trial of Prograf in conjunction with MMF and basiliximab, approximately 80% of patients maintained tacrolimus whole trough blood concentrations between 6-16 ng/mL during month 1-3 and, then, between 5-12 ng/mL from month 4 through 1 year.
The relative risks of toxicity and efficacy failure are related to tacrolimus whole blood trough concentrations. Therefore, monitoring of whole blood trough concentrations is recommended to assist in the clinical evaluation of toxicity and efficacy failure.
Heart Transplantation
Data from a European Phase 3 study indicate that trough concentrations of tacrolimus in whole blood, as measured by IMx® were most variable during the first week of dosing. From 1 week to 3 months post transplant, approximately 80% of patients maintained trough concentrations between 8-20 ng/mL and, from 3 months through 18 months post transplant, approximately 80% of patients maintained trough concentrations between 6-18 ng/mL.
The relative risk of toxicity; for example, nephrotoxicity and post-transplant diabetes mellitus, is increased with higher trough concentrations. Therefore, monitoring of whole blood trough concentrations is recommended to assist in the clinical evaluation of toxicity.
-
HOW SUPPLIED
Prograf capsules (tacrolimus capsules) strength 1 mg
(containing the equivalent of 1 mg anhydrous tacrolimus)
shape/color oblong/white
branding on capsule cap/body f
617
100 count bottle NDC: 21695-170-00 Made in Japan
Prograf injection (tacrolimus injection)
(for IV infusion only)
NDC: 0469-3016-01 Product Code 301601
5 mg/mL (equivalent of 5 mg of anhydrous tacrolimus per mL) supplied as a sterile solution in a 1 mL ampule, in boxes of 10 ampules
Made in Ireland
- REFERENCE
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PROGRAF
tacrolimus capsule, gelatin coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 21695-170(NDC:0469-0617) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength anhydrous tacrolimus (UNII: Y5L2157C4J) (tacrolimus - UNII:WM0HAQ4WNM) anhydrous tacrolimus 1 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) magnesium stearate (UNII: 70097M6I30) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) gelatin (UNII: 2G86QN327L) titanium dioxide (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (WHITE) Score no score Shape CAPSULE (CAPSULE) Size 11mm Flavor Imprint Code f;617;1;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 21695-170-00 1 in 1 CARTON 1 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050708 04/08/1994 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK
Trademark Results [Prograf]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PROGRAF 74177939 1867420 Live/Registered |
ASTELLAS PHARMA INC. 1991-06-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
