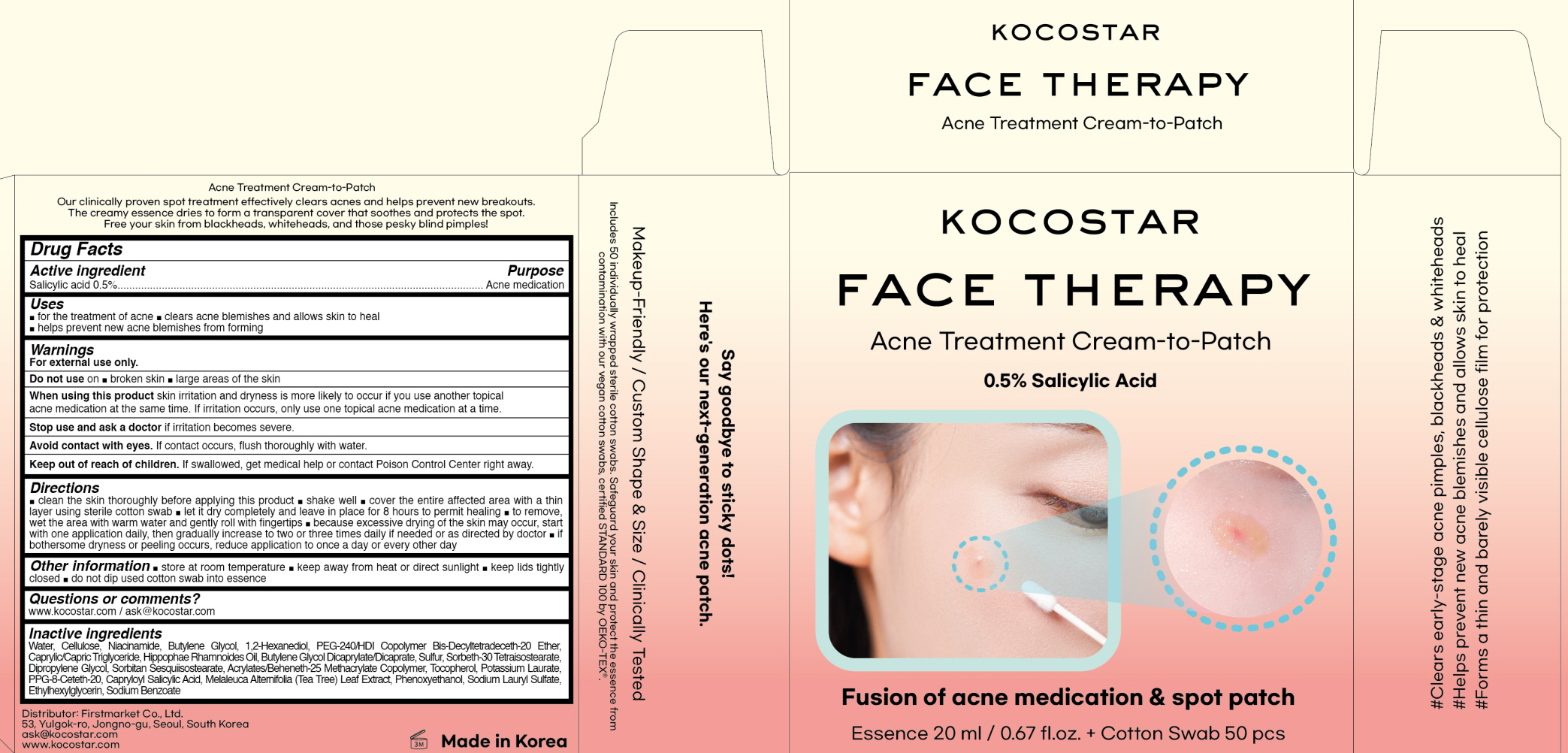
KOCOSTAR FACE THERAPY ACNE TREATMENT CREAM TO PATCH by FIRSTMARKET CO., LTD. / Natuzen Co.,Ltd.
KOCOSTAR FACE THERAPY ACNE TREATMENT CREAM TO PATCH by
Drug Labeling and Warnings
KOCOSTAR FACE THERAPY ACNE TREATMENT CREAM TO PATCH by is a Otc medication manufactured, distributed, or labeled by FIRSTMARKET CO., LTD., Natuzen Co.,Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
KOCOSTAR FACE THERAPY ACNE TREATMENT CREAM TO PATCH- salicylic acid liquid
FIRSTMARKET CO., LTD.
----------
INACTIVE INGREDIENT
Water, Cellulose, Niacinamide, Butylene Glycol, 1,2-Hexanediol, PEG-240/HDI Copolymer Bis-Decyltetradeceth-20 Ether, Caprylic/Capric Triglyceride, Hippophae Rhamnoides Oil, Butylene Glycol Dicaprylate/Dicaprate, Sulfur, Sorbeth-30 Tetraisostearate, Dipropylene Glycol, Sorbitan Sesquiisostearate, Acrylates/Beheneth-25 Methacrylate Copolymer, Tocopherol, Potassium Laurate, PPG-8-Ceteth-20, Capryloyl Salicylic Acid, Melaleuca Alternifolia (Tea Tree) Leaf Extract, Phenoxyethanol, Sodium Lauryl Sulfate, Ethylhexylglycerin, Sodium Benzoate
WARNINGS
For external use only.
Do not use on broken skin. large areas of the skin
When using this product skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Stop use and ask a doctor if irritation becomes severe.
Avoid contact with eyes. If contact occurs, flush thoroughly with water.
KEEP OUT OF REACH OF CHILDREN
If swallowed, get medical help or contact Poison Control Center right away.
Uses
for the treatment of acne
clears acne blemishes and allows skin to heal
helps prevent new acne blemishes from forming
Directions
clean the skin thoroughly before applying this productshake welcover the entire affected area with a thin layer using sterile cotton swablet it dry completely and leave in place for 8 hours to permit healingto remove, wet the area with warm water and gently roll with fingertips because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by doctorif bothersome dryness or peeling occurs, reduce application to once a day or every other day
| KOCOSTAR FACE THERAPY ACNE TREATMENT CREAM TO PATCH
salicylic acid liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - FIRSTMARKET CO., LTD. (688288012) |
| Registrant - FIRSTMARKET CO., LTD. (688288012) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Natuzen Co.,Ltd. | 688201272 | manufacture(83888-010) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.