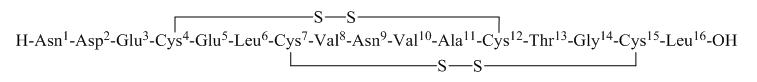TRULANCE IMMEDIATE RELEASE- plecanatide tablet
Trulance by
Drug Labeling and Warnings
Trulance by is a Prescription medication manufactured, distributed, or labeled by Synergy Pharmaceuticals Inc., UPM Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TRULANCE safely and effectively. See full prescribing information for TRULANCE.
TRULANCE (plecanatide) tablets, for oral use
Initial U.S. Approval: 2017WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
See full prescribing information for complete boxed warning.
- TRULANCE is contraindicated in patients less than 6 years of age; in young juvenile mice, plecanatide caused death due to dehydration. (4, 8.4)
- Avoid use of TRULANCE in patients 6 years to less than 18 years of age. (5.1, 8.4)
- The safety and effectiveness of TRULANCE have not been established in patients less than 18 years of age. (8.4)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
The recommended adult dosage of TRULANCE is
Administration Instructions (2.2):
- Take with or without food.
- Swallow tablets whole.
- For patients who have difficulty swallowing tablets whole or those with a nasogastric or gastric feeding tube, see full prescribing information with instructions for crushing the tablet and administering with applesauce or water.
DOSAGE FORMS AND STRENGTHS
Tablets: 3 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
Diarrhea: Patients may experience severe diarrhea. If severe diarrhea occurs, suspend dosing and rehydrate the patient. (5.2)
ADVERSE REACTIONS
Most common adverse reaction (≥2%) is diarrhea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Synergy Pharmaceuticals at 1-888-869-8869 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Preparation and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Serious Dehydration in Pediatric Patients
5.2 Diarrhea
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Chronic Idiopathic Constipation (CIC)
14.2 Irritable Bowel Syndrome with Constipation (IBS-C)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
- TRULANCE is contraindicated in patients less than 6 years of age; in nonclinical studies in young juvenile mice administration of a single oral dose of plecanatide caused deaths due to dehydration [see Contraindications (4), Use in Specific Populations (8.4)].
- Avoid use of TRULANCE in patients 6 years to less than 18 years of age [see Warnings and Precautions (5.1), Use in Specific Populations (8.4)].
- The safety and effectiveness of TRULANCE have not been established in patients less than 18 years of age [see Use in Specific Populations (8.4)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of TRULANCE for the treatment of CIC and IBS-C is 3 mg taken orally once daily.
2.2 Preparation and Administration Instructions
- Take TRULANCE with or without food [see Clinical Pharmacology (12.3)].
- If a dose is missed, skip the missed dose and take the next dose at the regular time. Do not take two doses at the same time.
- Swallow a tablet whole for each dose.
- For adult patients with swallowing difficulties, TRULANCE tablets can be crushed and administered orally either in applesauce or with water or administered with water via a nasogastric or gastric feeding tube. Mixing TRULANCE crushed tablets in other soft foods or in other liquids has not been tested.
Oral Administration in Applesauce:
- 1. In a clean container, crush the TRULANCE tablet to a powder and mix with 1 teaspoonful of room temperature applesauce.
- 2. Consume the entire tablet-applesauce mixture immediately. Do not store the mixture for later use.
Oral Administration in Water:
- 1. Place the TRULANCE tablet in a clean cup.
- 2. Pour approximately 30 mL of room temperature water into the cup.
- 3. Mix by gently swirling the tablet and water mixture for at least 10 seconds. The TRULANCE tablet will fall apart in the water.
- 4. Swallow the entire contents of the tablet water mixture immediately.
- 5. If any portion of the tablet is left in the cup, add another 30 mL of water to the cup, swirl for at least 10 seconds, and swallow immediately.
- 6. Do not store the tablet-water mixture for later use.
Administration with Water via a Nasogastric or Gastric Feeding Tube:
- 1. Place the TRULANCE tablet in a clean cup with 30 mL of room temperature water.
- 2. Mix by gently swirling the tablet and water mixture for at least 15 seconds. The TRULANCE tablet will fall apart in the water.
- 3. Flush the nasogastric or gastric feeding tube with 30 mL of water using a catheter tip syringe.
- 4. Draw up the mixture using the syringe and immediately administer via the nasogastric or gastric feeding tube. Do not reserve for future use.
- 5. If any portion of the tablet is left in the cup, add another 30 mL of water to the cup, swirl for at least 15 seconds, and using the same syringe, administer via the nasogastric or gastric feeding tube.
- 6. Using the same or a fresh syringe, flush the nasogastric or gastric feeding tube with at least 10 mL of water.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
TRULANCE is contraindicated in:
- Patients less than 6 years of age due to the risk of serious dehydration [see Warnings and Precautions (5.1), Use in Specific Populations (8.4)].
- Patients with known or suspected mechanical gastrointestinal obstruction.
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Serious Dehydration in Pediatric Patients
TRULANCE is contraindicated in patients less than 6 years of age. The safety and effectiveness of TRULANCE in patients less than 18 years of age have not been established. In young juvenile mice (human age equivalent of approximately 1 month to less than 2 years), plecanatide increased fluid-secretion into the intestines as a consequence of stimulation of guanylate cyclase-C (GC-C), resulting in mortality in some mice within the first 24 hours, apparently due to dehydration. Due to increased intestinal expression of GC-C, patients less than 6 years of age may be more likely than patients 6 years of age and older to develop severe diarrhea and its potentially serious consequences.
Avoid the use of TRULANCE in patients 6 years to less than 18 years of age. Although there were no deaths in older juvenile mice, given the deaths in younger mice and the lack of clinical safety and efficacy data in pediatric patients, avoid the use of TRULANCE in patients 6 years to less than 18 years of age [see Contraindications (4), Warnings and Precautions (5.2), Use in Specific Populations (8.4)].
5.2 Diarrhea
Diarrhea was the most common adverse reaction in four placebo-controlled clinical trials, two in patients with CIC and two in patients with IBS-C. Severe diarrhea was reported in 0.6% of patients in two trials in patients with CIC and in 0.6% of patients in the two trials in patients with IBS-C [see Adverse Reactions (6.1)]. If severe diarrhea occurs, suspend dosing and rehydrate the patient.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Demographic characteristics were comparable between the TRULANCE and placebo groups in all studies [see Clinical Studies (14)].
Chronic Idiopathic Constipation (CIC)
The safety data described below reflect data from 1733 adult patients with CIC randomized in two double-blind, placebo-controlled clinical trials (Study 1 and Study 2) to receive placebo or 3 mg of TRULANCE once daily for 12 weeks.
Most Common Adverse Reactions
Table 1 provides the incidence of adverse reactions reported in at least 2% of CIC patients in the TRULANCE-treated group and at an incidence that was greater than in the placebo group.
Table 1: Most Common Adverse Reactionsa in Two Placebo-Controlled Trials of TRULANCE [Study 1 and Study 2] in Patients with CIC Adverse Reaction TRULANCE, 3 mg
(N = 863)
%Placebo
(N = 870)
%a: Reported in at least 2% of TRULANCE-treated patients with CIC and at an incidence greater than placebo. Diarrhea
5
1
Diarrhea
The majority of reported cases of diarrhea occurred within 4 weeks of treatment initiation. Severe diarrhea was reported in 0.6% of TRULANCE-treated patients compared to 0.3% of placebo-treated patients. Severe diarrhea was reported to occur within the first 3 days of treatment [see Warnings and Precautions (5.2)].
Adverse Reactions Leading to Discontinuation
Discontinuations due to adverse reactions occurred in 4% of TRULANCE-treated patients and 2% of placebo-treated patients. The most common adverse reaction leading to discontinuation was diarrhea: 2% of TRULANCE-treated patients and 0.5% of placebo-treated patients withdrew due to diarrhea.
Less Common Adverse Reactions
Adverse reactions reported in less than 2% of TRULANCE-treated patients and at an incidence greater than placebo were: sinusitis, upper respiratory tract infection, abdominal distension, flatulence, abdominal tenderness, and increased liver biochemical tests (2 patients with alanine aminotransferase (ALT) greater than 5 to 15 times the upper limit of normal and 3 patients with aspartate aminotransferase (AST) greater than 5 times the upper limit of normal).
Irritable Bowel Syndrome with Constipation (IBS-C)
The safety data described below reflect data from 1449 adult patients with IBS-C randomized in two double-blind, placebo-controlled clinical trials (Study 3 and Study 4) to receive placebo or 3 mg TRULANCE once daily for 12 weeks.
Most Common Adverse Reactions
Table 2 provides the incidence of adverse reactions reported in at least 2% of IBS-C patients treated with TRULANCE and at an incidence that was greater than in the placebo group.
Table 2: Most Common Adverse Reactionsa in Two Placebo-Controlled Trials of TRULANCE [Study 3 and Study 4] in Patients with IBS-C Adverse Reaction TRULANCE, 3 mg
(N = 723)
%Placebo
(N = 726)
%a: Reported in at least 2% of TRULANCE-treated patients with IBS-C and at an incidence greater than placebo. b: Verbatim reports of diarrhea were recorded as adverse reactions; reports of loose stools and increase in stool frequency were recorded as adverse reactions if they were also reported to be bothersome to the patient. Diarrheab
4.3
1
Diarrhea
The majority of reported cases of diarrhea occurred within 4 weeks of treatment initiation. Severe diarrhea was reported in 1% of TRULANCE-treated patients compared to 0.1% of placebo-treated patients [see Warnings and Precautions (5.2)]. Severe diarrhea was reported to occur within the first day of treatment.
Adverse Reactions Leading to Discontinuation
Discontinuations due to adverse reactions occurred in 2.5% of TRULANCE-treated patients and 0.4% of placebo-treated patients. The most common adverse reaction leading to discontinuation was diarrhea: 1.2% of TRULANCE-treated patients and 0% of placebo-treated patients withdrew due to diarrhea.
Less Common Adverse Reactions
Adverse reactions reported in 1% or more but less than 2% of TRULANCE-treated patients and at an incidence greater than placebo were: nausea, nasopharyngitis, upper respiratory tract infection, urinary tract infection, and dizziness. Two patients reported increased liver biochemical tests (alanine aminotransferase (ALT) greater than 5 to 15 times the upper limit of normal).
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Plecanatide and its active metabolite are negligibly absorbed systemically following oral administration [see Clinical Pharmacology (12.3)] and maternal use is not expected to result in fetal exposure to the drug. The available data on TRULANCE use in pregnant women are not sufficient to inform any drug-associated risks for major birth defects and miscarriage. In animal developmental studies, no effects on embryo-fetal development were observed with oral administration of plecanatide in mice and rabbits during organogenesis at doses much higher than the recommended human dosage.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the United States general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Pregnant mice and rabbits were administered plecanatide during the period of organogenesis. There was no evidence of harm to embryo-fetal development at oral doses up to 800 mg/kg/day in mice and 250 mg/kg/day in rabbits. Oral administration of up to 600 mg/kg/day in mice during organogenesis through lactation produced no developmental abnormalities or effects on growth, learning and memory, or fertility in the offspring through maturation.
The maximum recommended human dose is approximately 0.05 mg/kg/day, based on a 60-kg body weight. Limited systemic exposure to plecanatide was achieved in animals during organogenesis (area under the plasma concentration-time curve (AUCt) = 449 ng∙h/mL in rabbits given 250 mg/kg/day). Plecanatide and its active metabolite are not measurable in human plasma following administration of the recommended clinical dosage. Therefore, animal and human doses should not be compared directly for evaluating relative exposure.
8.2 Lactation
Risk Summary
There is no information regarding the presence of plecanatide in human milk, or its effects on milk production or the breastfed infant. No lactation studies in animals have been conducted. Plecanatide and its active metabolite are negligibly absorbed systemically following oral administration [see Clinical Pharmacology (12.3)].
It is unknown whether the negligible systemic absorption of plecanatide by adults will result in a clinically relevant exposure to breastfed infants. Exposure to plecanatide in breastfed infants has the potential for serious adverse effects [see Use in Special Populations (8.4)]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for TRULANCE and any potential adverse effects on the breastfed infant from TRULANCE or from the underlying maternal condition.
8.4 Pediatric Use
TRULANCE is contraindicated in pediatric patients less than 6 years of age. Avoid use of TRULANCE in patients 6 years to less than 18 years of age [see Contraindications (4), Warnings and Precautions (5.1)]. The safety and effectiveness of TRULANCE in patients less than 18 years of age have not been established.
In nonclinical studies, deaths occurred within 24 hours in young juvenile mice (human age equivalent of approximately 1 month to less than 2 years) following oral administration of plecanatide, as described below in Juvenile Animal Toxicity Data. Because of increased intestinal expression of GC-C, patients less than 6 years of age may be more likely than patients 6 years of age and older to develop diarrhea and its potentially serious consequences. TRULANCE is contraindicated in patients less than 6 years of age. Given the deaths in young juvenile mice and the lack of clinical safety and efficacy data in pediatric patients, avoid the use of TRULANCE in patients 6 years to less than 18 years of age.
Juvenile Animal Toxicity Data
Single oral doses of plecanatide at 0.5 mg/kg and 10 mg/kg caused mortality in young juvenile mice on postnatal days 7 and 14, respectively (human age equivalent of approximately 1 month to less than 2 years). Treatment-related increases in the weight of intestinal contents were observed in juvenile mice following single doses of plecanatide on postnatal day 14 (human age equivalent of approximately less than 2 years), consistent with increased fluid in the intestinal lumen. Although the recommended human dose is approximately 0.05 mg/kg/day, based on a 60-kg body weight, plecanatide and its active metabolite are not measurable in adult human plasma, whereas systemic absorption was demonstrated in the juvenile animal toxicity studies. Animal and human doses should not be compared directly for evaluating relative exposure.
8.5 Geriatric Use
Chronic Idiopathic Constipation (CIC)
Of 2601 subjects in placebo-controlled clinical trials of TRULANCE, 273 (10%) were 65 years of age and over, and 47 (2%) were 75 years and over. Clinical studies of TRULANCE did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from patients 18 years to less than 65 years of age.
Irritable Bowel Syndrome with Constipation (IBS-C)
Of 1621 subjects in the placebo-controlled clinical studies of TRULANCE, 134 (8.3%) were 65 years of age and over, and 25 (1.5%) were 75 years and over. Clinical studies of TRULANCE did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from patients 18 years to less than 65 years of age.
-
11 DESCRIPTION
TRULANCE (plecanatide) is a guanylate cyclase-C (GC-C) agonist. Plecanatide is a 16 amino acid peptide with the following chemical name: L-Leucine, L-asparaginyl-L-α-aspartyl-L-α-glutamyl-L-cysteinyl-L-α-glutamyl-L-leucyl-L-cysteinyl-L-valyl-L-asparaginyl-L-valyl-L-alanyl-L-cysteinyl-L-threonylglycyl-L-cysteinyl-, cyclic (4→12),(7→15)-bis(disulfide).
The molecular formula of plecanatide is C65H104N18O26S4 and the molecular weight is 1682 Daltons. The amino acid sequence for plecanatide is shown below:
The solid lines linking cysteines illustrate disulfide bridges.
Plecanatide is an amorphous, white to off-white powder. It is soluble in water. TRULANCE tablets are supplied as 3 mg tablets for oral administration. The inactive ingredients are magnesium stearate and microcrystalline cellulose.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Plecanatide is a structural analog of human uroguanylin, and similarly to uroguanylin, plecanatide functions as a guanylate cyclase-C (GC-C) agonist. Both plecanatide and its active metabolite bind to GC-C and act locally on the luminal surface of the intestinal epithelium. Activation of GC-C results in an increase in both intracellular and extracellular concentrations of cyclic guanosine monophosphate (cGMP). Elevation of extracellular cGMP has been associated with a decrease in the activity of pain-sensing nerves in animal models of visceral pain. Elevation of intracellular cGMP stimulates secretion of chloride and bicarbonate into the intestinal lumen, mainly through activation of the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel, resulting in increased intestinal fluid and accelerated transit. In animal models, plecanatide has been shown to increase fluid secretion into the gastrointestinal (GI) tract, accelerate intestinal transit, and cause changes in stool consistency.
In an animal model of visceral pain, plecanatide reduced abdominal muscle contractions, a measure of intestinal pain.
12.2 Pharmacodynamics
Food Effect
Subjects who received either a low-fat, low calorie (LF-LC) meal or a high fat, high calorie (HF-HC) meal reported looser stools than fasted subjects up to 24 hours after a single dose of TRULANCE 9 mg (3 times the recommended dose). In clinical studies, TRULANCE was administered with or without food [see Dosage and Administration (2.2)].
12.3 Pharmacokinetics
Absorption
Plecanatide was minimally absorbed with negligible systemic availability following oral administration. Concentrations of plecanatide and its active metabolite in plasma were below the limit of quantitation in the majority of analyzed plasma samples after an oral TRULANCE dose of 3 mg. Therefore, standard pharmacokinetic parameters such as AUC, maximum concentration (Cmax), and half-life (t½) could not be calculated.
Food Effect
In a crossover study, 24 healthy subjects were given a single dose of TRULANCE 9 mg (3 times the recommended dose) in 3 different states: fasted; following a low-fat, low-calorie meal (LF-LC; approximately 350 calories: 17% from fat, 66% from carbohydrate, and 17% from protein); and following a high-fat, high-calorie meal (HF-HC; approximately 1000 calories: 60% from fat, 25% from carbohydrate, and 15% from protein). Plecanatide was detected in 1 subject (fasted state) at 0.5 and 1 hour post dose. Plecanatide concentrations were below the limit of quantitation for all other time points and for all other subjects. The active metabolite was not detected in any subject.
Distribution
Given that plecanatide concentrations following clinically relevant oral doses were not measurable, plecanatide is expected to be minimally distributed in tissues. Oral plecanatide was localized to the GI tract where it exerted its effects as a GC-C agonist with negligible systemic exposure. Plecanatide exhibited little to no binding to human serum albumin or human α-1-acid glycoprotein.
Elimination
Drug Interaction Studies
Neither plecanatide nor its active metabolite inhibited the cytochrome P450 (CYP) enzymes 2C9 and 3A4, and they did not induce CYP3A4 in vitro.
Plecanatide and its active metabolite were neither substrates nor inhibitors of the transporters P-glycoprotein (P-gp) or breast cancer resistance protein (BCRP) in vitro.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
The carcinogenic potential of plecanatide was assessed in 2-year carcinogenicity studies in mice and rats. Plecanatide was not tumorigenic in mice at oral doses up to 90 mg/kg/day or in rats at oral doses up to 100 mg/kg/day. Limited systemic exposure to plecanatide was achieved at the tested dose levels in animals, whereas no detectable exposure occurred in humans. Therefore, animal and human doses should not be compared directly for evaluating relative exposure.
-
14 CLINICAL STUDIES
14.1 Chronic Idiopathic Constipation (CIC)
The efficacy of TRULANCE for the management of symptoms of CIC was established in two 12-week, double-blind, placebo-controlled, randomized, multicenter clinical studies in adult patients (Study 1 and Study 2). In the Intention-to-Treat (ITT) population, a total of 905 patients (Study 1) and 870 patients (Study 2) were randomized 1:1 to either placebo or TRULANCE 3 mg, once daily. In clinical studies, study medication was administered without respect to food intake. Demographics for these studies included an overall mean age of 45 years (range 18 to 80 years), 80% female, 72% white, and 24% black.
To be eligible for the studies, patients were required to meet modified Rome III criteria for at least 3 months prior to the screening visit, with symptom onset for at least 6 months prior to diagnosis. Rome III criteria were modified to require that patients report less than 3 defecations per week, rarely have a loose stool without the use of laxatives, not use manual maneuvers to facilitate defecations, and not meet criteria for IBS-C. In addition, patients were required to report at least two of the following symptoms:
- Straining during at least 25% of defecations
- Lumpy or hard stool in at least 25% of defecations
- Sensation of incomplete evacuations for at least 25% of defecations
- Sensation of anorectal obstruction/blockage for at least 25% of defecations
Patients who met these criteria were also required to demonstrate the following during the last 2 weeks of the screening period:
- Less than 3 complete spontaneous bowel movements (CSBMs) (a CSBM is an SBM that is associated with a sense of complete evacuation) in each of the two weeks
- Bristol Stool Form Scale (BSFS) of 6 or 7 in less than 25% of spontaneous bowel movements (SBMs) (an SBM is a bowel movement occurring in the absence of laxative use)
-
One out of the following three:
- BSFS of 1 or 2 in at least 25% of defecations
- A straining value recorded on at least 25% of days when a BM was reported
- At least 25% of BMs result in a sense of incomplete evacuation
The efficacy of TRULANCE was assessed using a responder analysis and change-from-baseline in CSBM and SBM endpoints. Efficacy was assessed using information provided by patients on a daily basis in an electronic diary.
A responder was defined as a patient who had at least 3 CSBMs in a given week and an increase of at least 1 CSBM from baseline in the same week for at least 9 weeks out of the 12 week treatment period and at least 3 of the last 4 weeks of the study. The responder rates are shown in Table 3.
Table 3: Efficacy Responder Rates in the Two Placebo-controlled Studies of CIC: at least 9 of 12 weeks and at least 3 of the last 4 weeks (ITT Population) a: p-value <0.005 b: CI = confidence interval c: Primary endpoint defined as a patient who had a least 3 CSBMs in a given week and an increase of at least 1 CSBM from baseline in the same week for at least 9 weeks out of the 12 week treatment period and at least 3 of the last 4 weeks of the study. Study 1
TRULANCE 3 mg
N = 453Placebo
N = 452Treatment Differencea
[95% CIb]Responderc
21%
10%
11%
[6.1%, 15.4%]Study 2
TRULANCE 3 mg
N = 430Placebo
N = 440Treatment Differencea
[95% CIb]Responderc
21%
13%
8%
[2.6%, 12.4%]In both studies, improvements in the frequency of CSBMs/week were seen as early as week 1 with improvement maintained through week 12. The difference between the TRULANCE group and the placebo group in the mean change of CSBMs/week frequency from baseline to week 12 was approximately 1.1 CSBMs/week.
Over the 12 week treatment period, improvements were observed in stool frequency (number of CSBMs/week and SBMs/week) and/or stool consistency (as measured by the BSFS), and/or in the amount of straining with bowel movements (amount of time pushing or physical effort to pass stool) in the TRULANCE group as compared to placebo.
Following completion of the study drug treatment period, patients continued to record data in the daily diary for a 2 week Post-Treatment Period. During this time, TRULANCE-treated patients generally returned to baseline for these study endpoints.
In Studies 1 and 2, a third randomized treatment arm of TRULANCE 6 mg once daily did not demonstrate additional treatment benefit and had a greater incidence of adverse reactions than TRULANCE 3 mg once daily. Therefore, TRULANCE 6 mg once daily is not recommended [see Dosage and Administration (2.1)].
14.2 Irritable Bowel Syndrome with Constipation (IBS-C)
The efficacy of TRULANCE for the management of symptoms of IBS-C was established in two 12-week, double-blind, placebo-controlled, randomized, multicenter clinical studies in adult patients (Study 3 and Study 4). In the Intention-to-Treat (ITT) population, a total of 699 patients (Study 3) and 754 patients (Study 4) received treatment with placebo or TRULANCE 3 mg once daily. In clinical studies, study medication was administered without respect to food intake. Demographics for these studies included an overall mean age of 44 years (range 18 to 83 years), 74% female, 73% white, and 22% black.
To be eligible, patients were required to meet the Rome III criteria for IBS for at least 3 months prior to the screening visit, with symptom onset for at least 6 months prior to diagnosis. Diagnosis required recurrent abdominal pain or discomfort at least 3 days/month in the last 3 months associated with 2 or more of 1) improvement with defecation, 2) onset associated with a change in frequency of stool, and 3) onset associated with a change in form (appearance) of stool. Patients also met the IBS-C differentiation criteria for constipation, characterized by a stool pattern such that at least 25% of defecations are hard or lumpy stools and no more than 25% of defecations are loose or watery stool.
Patients who met these criteria were excluded if they demonstrated the following during the last 2 weeks of the screening period:
- Worst abdominal pain intensity (WAPI) score of 0 on an 11-point scale for more than 2 days during each week
- An average WAPI of less than 3 for either week
- More than 3 complete spontaneous bowel movements (CSBMs) or more than six spontaneous bowel movements (SBMs) per week in either week
- Bristol Stool Form Scale (BSFS) of 7 for any SBM recorded
- More than 1 day in either week with a BSFS of 6 for any SBM recorded
- No use of rescue laxative (bisacodyl) within 72 hours before randomization
The efficacy of TRULANCE was assessed using a responder analysis based on abdominal pain intensity and a stool frequency responder (CSBM) endpoint. Efficacy was assessed using information provided by patients on a daily basis through an electronic phone diary system.
A responder was defined as a patient who met both the abdominal pain intensity and stool frequency responder criteria in the same week for at least 6 of the 12 treatment weeks. The abdominal pain intensity and stool frequency responder criteria assessed each week were defined as:
- Abdominal pain intensity responder: a patient who experienced a decrease in the weekly average of worst abdominal pain in the past 24 hours score (measured daily) of at least 30% compared with baseline weekly average.
- Stool frequency responder: a patient who experienced an increase of at least 1 CSBM per week from baseline.
The responder rates are shown in Table 4.
Table 4: Efficacy Responder Rates in the Two Placebo-controlled Studies of IBS-C: Overall Responder for at least 6 of the 12 Treatment Weeks (ITT Population) a: CI = confidence interval b: A responder for these trials was defined as a patient who met both the abdominal pain and CSBM weekly responder criteria for at least 6 of the 12 weeks. c: An abdominal pain responder was defined as a patient who met the criteria of at least 30% reduction from baseline in weekly average of the worst daily abdominal pain, for at least 6 of the 12 weeks. d: A CSBM responder was defined as a patient who achieved an increase in at least 1 CSBM per week, from baseline, for at least 6 of 12 weeks. Study 3
Placebo
N = 350TRULANCE 3 mg
N = 349Treatment Difference
[95% CIa]Responderb
18%
30%
12%
[6%, 18%]Components of Responder Endpoint
Abdominal Pain Responderc
32%
41%
CSBM Responderd
35%
48%
Study 4
Placebo
N = 379TRULANCE 3 mg
N = 375Treatment Difference
[95% CIa]Responderb
14%
21%
7%
[2%, 13%]Components of Responder Endpoint
Abdominal Pain Responderc
23%
33%
CSBM Responderd
28%
34%
In both studies, the proportion of responders who were also weekly responders for at least 2 of the 4 treatment weeks in month 3, the last month of treatment was greater in the TRULANCE groups compared to placebo.
Over the 12 week treatment period, improvements were observed in both stool consistency (as measured by the BSFS) and in the amount of straining with bowel movements (amount of time pushing or physical effort to pass stool) in the 3 mg TRULANCE group as compared to placebo.
Following completion of the study drug treatment period, patients continued to record data in the daily diary for a 2-week Post-Treatment Period. During this time, TRULANCE-treated patients generally returned to baseline for these study endpoints.
In Studies 3 and 4, a third randomized treatment arm of TRULANCE 6 mg once daily did not demonstrate additional treatment benefit over the 3 mg dose. Therefore, TRULANCE 6 mg once daily is not recommended [see Dosage and Administration (2.1)].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
TRULANCE tablets are packaged in an aluminum foil unit dose blister pack of 30 in a child-resistant pack or in a white, opaque, high-density polyethylene round bottle with a screw-top polypropylene child-resistant cap and heat-activated induction seal. Each bottle container-closure system also contains a desiccant and a polyester coil.
TRULANCE 3 mg tablets are white to off-white, plain and round, debossed with "SP" on one side and "3" for 3 mg on the other side and supplied as:
NDC Number Size 70194-203-30
Bottle of 30
70194-003-30
Aluminum foil unit dose blister pack of 30 in a child-resistant pack
Store at room temperature, 20 to 25°C (68 to 77°F); excursions permitted to 15 to 30°C (59 to 86°F) [see USP Controlled Room Temperature].
Keep TRULANCE in a dry place. Protect from moisture. For bottles, keep TRULANCE in the original bottle. Do not remove desiccant from the bottle. Do not subdivide or repackage.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Advise Patients:
Diarrhea
To stop TRULANCE and contact their healthcare provider if they experience severe diarrhea [see Warnings and Precautions (5.2)].
Accidental Ingestion
Accidental ingestion of TRULANCE in children, especially in children less than 6 years of age, may result in severe diarrhea and dehydration. Instruct patients to take steps to store TRULANCE securely and out of reach of children and to dispose of unused TRULANCE [see Contraindications (4), Warnings and Precautions (5.2)].
Administration and Handling Instructions
- To take TRULANCE once daily with or without food [see Dosage and Administration (2.2)].
- If a dose is missed, skip the missed dose and take the next dose at the regular time. Do not take two doses at the same time.
- To swallow TRULANCE tablets whole.
- If adult patients have swallowing difficulties, TRULANCE tablets can be crushed and administered orally in either applesauce or with water, or administered with water via a nasogastric or gastric feeding tube, as described in the Medication Guide.
- To keep TRULANCE in a dry place. Protect from moisture. For bottles, keep TRULANCE in the original bottle. Do not remove desiccant from the bottle. Do not subdivide or repackage. Remove and discard polyester coil after opening. Keep bottles closed tightly [see How Supplied/Storage and Handling (16)].
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
Medication Guide
TRULANCE® (TROO lans)
(plecanatide) tabletsThis Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 02/2018 What is the most important information I should know about TRULANCE?
- Do not give TRULANCE to children who are less than 6 years of age. It may harm them.
- You should not give TRULANCE to children 6 years to less than 18 years of age. It may harm them.
- See " What are the possible side effects of TRULANCE? " for more information about side effects.
What is TRULANCE?
TRULANCE is a prescription medicine used in adults to treat:- a type of constipation called chronic idiopathic constipation (CIC). Idiopathic means the cause of the constipation is unknown.
- irritable bowel syndrome with constipation (IBS-C).
It is not known if TRULANCE is safe and effective in children less than 18 years of age.
Who should not take TRULANCE?
- Do not give TRULANCE to children who are less than 6 years of age.
- Do not take TRULANCE if a doctor has told you that you have a bowel blockage (intestinal obstruction).
Before taking TRULANCE, tell your doctor about all of your medical conditions, including if you:
- are pregnant or plan to become pregnant. It is not known if TRULANCE will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if TRULANCE passes into your breast milk. Talk with your doctor about the best way to feed your baby if you take TRULANCE.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take TRULANCE?
- Take TRULANCE exactly as your doctor tells you to take it.
- Take TRULANCE by mouth, 1 time each day with or without food.
- If you miss a dose, skip the missed dose. Take the next dose at your regular time. Do not take 2 doses at the same time.
-
TRULANCE tablets should be swallowed whole.
- Adults who cannot swallow TRULANCE tablets whole may crush the TRULANCE tablet and mix with applesauce or dissolve TRULANCE in water before swallowing. TRULANCE tablets may also be taken with water by adults through a nasogastric or gastric feeding tube.
-
It is not known if TRULANCE is safe and effective when crushed and mixed with other foods or dissolved in other liquids.
Taking TRULANCE in applesauce:
- Crush the TRULANCE tablet in a clean container until it is a powder and mix with 1 teaspoon of room temperature applesauce.
- Swallow all of the TRULANCE and applesauce mixture right away. Do not keep the TRULANCE and applesauce mixture for future use.
Taking TRULANCE in water:
- Place the TRULANCE tablet in a clean cup and pour 1 ounce (30 mL) of room temperature water into the cup.
- Gently swirl the TRULANCE tablet and water for at least 10 seconds. The TRULANCE tablet will fall apart in the water.
- Swallow all of the TRULANCE tablet and water mixture right away. Do not keep the mixture for future use.
- If you see any part of the tablet left in the cup, add another 1 ounce (30 mL) of water to the cup, swirl for at least 10 seconds, and swallow right away.
Taking TRULANCE through a nasogastric or gastric feeding tube:
Gather the supplies you will need to take your TRULANCE dose. Your doctor should tell you what size catheter tip syringe you will need for your dose. Ask your doctor if you have any questions about how to give TRULANCE the right way.- Place the TRULANCE tablet in a clean cup with 1 ounce (30 mL) of room temperature water.
- Gently swirl the TRULANCE tablet and water for at least 15 seconds. The TRULANCE tablet will fall apart in the water.
- Flush the nasogastric or gastric feeding tube with 1 ounce (30 mL) of water.
- Draw up the TRULANCE tablet and water mixture into a catheter tip syringe and give right away through the nasogastric or gastric feeding tube. Do not keep the mixture for future use.
- If you see any part of the tablet left in the cup, add another 1 ounce (30 mL) of water to the cup, swirl for at least 15 seconds and use the same catheter tip syringe to give the mixture through the nasogastric or gastric feeding tube.
- Using the same or another catheter tip syringe, flush the nasogastric or gastric feeding tube with at least 10 mL of water.
-
What are the possible side effects of TRULANCE?
TRULANCE can cause serious side effects, including: - See "What is the most important information I should know about TRULANCE?"
-
Diarrhea is the most common side effect of TRULANCE, and it can sometimes be severe.
- Diarrhea often begins within the first 4 weeks of TRULANCE treatment.
-
Stop taking TRULANCE and call your doctor if you develop severe diarrhea.
These are not all the possible side effects of TRULANCE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store TRULANCE?
- Store TRULANCE at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep TRULANCE in a secure place and in the bottle or blister pack that it comes in.
- The TRULANCE bottle contains a desiccant packet to help keep your medicine dry (protect it from moisture). Do not remove the desiccant packet from the bottle.
- The TRULANCE bottle contains a polyester coil to help protect the tablets during shipping. Remove the polyester coil from the bottle and throw it away after opening the bottle.
- Keep the container of TRULANCE tightly closed and in a dry place.
- Safely throw away TRULANCE that is out of date or no longer needed.
Keep TRULANCE and all medicines out of the reach of children.
-
General information about the safe and effective use of TRULANCE.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use TRULANCE for a condition for which it was not prescribed. Do not give TRULANCE to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your doctor or pharmacist for information about TRULANCE that is written for health professionals.
What are the ingredients in TRULANCE?
Active ingredient: plecanatide
Inactive ingredients: magnesium stearate and microcrystalline cellulose
TRULANCE® is a registered trademark of Synergy Pharmaceuticals Inc.
Manufactured for: Synergy Pharmaceuticals Inc. 420 Lexington Avenue, Suite 2012 New York, New York 10170
©2016 Synergy Pharmaceuticals Inc. All rights reserved.
For more information, go to www.synergypharma.com or call 1-888-869-8869. -
PRINCIPAL DISPLAY PANEL - 3 mg Tablet Blister Pack Carton
NDC: 70194-003-30
Trulance®
(plecanatide) tablets3 mg
ATTENTION PHARMACIST:
Dispense the accompanying
Medication Guide to each patient.Rx Only 30 Tablets
KEEP OUT OF REACH OF CHILDREN.
Dosage and Administration:
One tablet once daily.
See full Prescribing Information.Each tablet contains 3 mg plecanatide
Store at room temperature, 20-25°C
(68-77°F); excursions permitted to
15-30°C (59-86°F) [USP Controlled
Room Temperature]. -
INGREDIENTS AND APPEARANCE
TRULANCE IMMEDIATE RELEASE
plecanatide tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70194-003 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Plecanatide (UNII: 7IK8Z952OK) (Plecanatide - UNII:7IK8Z952OK) Plecanatide 3 mg Inactive Ingredients Ingredient Name Strength Magnesium Stearate (UNII: 70097M6I30) Microcrystalline Cellulose (UNII: OP1R32D61U) Product Characteristics Color WHITE Score no score Shape ROUND Size 6mm Flavor Imprint Code SP;3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70194-003-30 1 in 1 CARTON 02/21/2017 1 30 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208745 02/21/2017 Labeler - Synergy Pharmaceuticals Inc. (827655445) Establishment Name Address ID/FEI Business Operations UPM Pharmaceuticals 032125469 MANUFACTURE(70194-003)
Trademark Results [Trulance]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TRULANCE 87392678 5349314 Live/Registered |
BAUSCH HEALTH IRELAND LIMITED 2017-03-30 |
 TRULANCE 87042213 5242709 Live/Registered |
BAUSCH HEALTH IRELAND LIMITED 2016-05-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.