JAKAFI- ruxolitinib tablet
JAKAFI by
Drug Labeling and Warnings
JAKAFI by is a Prescription medication manufactured, distributed, or labeled by Incyte Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use JAKAFI safely and effectively. See full prescribing information for JAKAFI.
JAKAFI® (ruxolitinib) tablets, for oral use
Initial U.S. Approval: 2011INDICATIONS AND USAGE
Jakafi is a kinase inhibitor indicated for treatment of:
- intermediate or high-risk myelofibrosis, including primary myelofibrosis, post-polycythemia vera myelofibrosis and post-essential thrombocythemia myelofibrosis in adults. (1.1)
- polycythemia vera in adults who have had an inadequate response to or are intolerant of hydroxyurea. (1.2)
- steroid-refractory acute graft-versus-host disease in adult and pediatric patients 12 years and older (1.3)
DOSAGE AND ADMINISTRATION
Doses should be individualized based on safety and efficacy. Starting doses per indication are noted below.
Myelofibrosis (2.1)
- The starting dose of Jakafi is based on patient’s baseline platelet count:
Greater than 200 X 109/L: 20 mg given orally twice daily
100 X 109/L to 200 X 109/L: 15 mg given orally twice daily
50 X 109/L to less than 100 X 109/L: 5 mg given orally twice daily - Monitor complete blood counts every 2 to 4 weeks until doses are stabilized, and then as clinically indicated. Modify or interrupt dosing for thrombocytopenia.
Polycythemia Vera (2.2)
- The starting dose of Jakafi is 10 mg given orally twice daily.
Acute Graft-Versus-Host Disease (2.3)
-
The starting dose of Jakafi is 5 mg given orally twice daily.
DOSAGE FORMS AND STRENGTHS
Tablets: 5 mg, 10 mg, 15 mg, 20 mg and 25 mg. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Thrombocytopenia, Anemia and Neutropenia: Manage by dose reduction, or interruption, or transfusion. (5.1)
- Risk of Infection: Assess patients for signs and symptoms of infection and initiate appropriate treatment promptly. Serious infections should have resolved before starting therapy with Jakafi. (5.2)
- Symptom Exacerbation Following Interruption or Discontinuation: Manage with supportive care and consider resuming treatment with Jakafi. (5.3)
- Risk of Non-Melanoma Skin Cancer: Perform periodic skin examinations. (5.4)
- Lipid Elevations: Assess lipid levels 8-12 weeks from start of therapy and treat as needed. (5.5)
ADVERSE REACTIONS
- In myelofibrosis and polycythemia vera, the most common hematologic adverse reactions (incidence > 20%) are thrombocytopenia and anemia. The most common nonhematologic adverse reactions (incidence ≥15%) are bruising, dizziness, headache, and diarrhea. (6.1 and 6.2)
- In acute graft-versus-host disease, the most common hematologic adverse reactions (incidence > 50%) are anemia, thrombocytopenia, and neutropenia. The most common nonhematologic adverse reactions (incidence > 50%) are infections and edema. (6.3)
To report SUSPECTED ADVERSE REACTIONS, contact Incyte Corporation at 1-855-463-3463 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
RECENT MAJOR CHANGES
1. INDICATIONS AND USAGE
1.1 Myelofibrosis
1.2 Polycythemia Vera
1.3 Acute Graft-Versus-Host Disease
2. DOSAGE AND ADMINISTRATION
2.1 Myelofibrosis
2.2 Polycythemia Vera
2.3 Acute Graft-Versus-Host Disease
2.4 Dose Modifications for Concomitant Use with Strong CYP3A4 Inhibitors or Fluconazole
2.5 Dose Modifications for Renal or Hepatic Impairment
2.6 Method of Administration
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1 Thrombocytopenia, Anemia and Neutropenia
5.2 Risk of Infection
5.3 Symptom Exacerbation Following Interruption or Discontinuation of Treatment with Jakafi
5.4 Non-Melanoma Skin Cancer
5.5 Lipid Elevations
6. ADVERSE REACTIONS
6.1 Clinical Trials Experience in Myelofibrosis
6.2 Clinical Trial Experience in Polycythemia Vera
6.3 Clinical Trial Experience in Acute Graft-Versus-Host Disease
7. DRUG INTERACTIONS
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10. OVERDOSAGE
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14. CLINICAL STUDIES
14.1 Myelofibrosis
14.2 Polycythemia Vera
14.3 Acute Graft-Versus-Host Disease
16. HOW SUPPLIED/STORAGE AND HANDLING
17. PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1. INDICATIONS AND USAGE
1.1 Myelofibrosis
Jakafi is indicated for treatment of intermediate or high-risk myelofibrosis (MF), including primary MF, post-polycythemia vera MF and post-essential thrombocythemia MF in adults.
-
2. DOSAGE AND ADMINISTRATION
2.1 Myelofibrosis
The recommended starting dose of Jakafi is based on platelet count (Table 1). A complete blood count (CBC) and platelet count must be performed before initiating therapy, every 2 to 4 weeks until doses are stabilized, and then as clinically indicated [see Warnings and Precautions (5.1)]. Doses may be titrated based on safety and efficacy.
Table 1: Jakafi Starting Doses for Myelofibrosis Platelet Count Starting Dose Greater than 200 X 109/L 20 mg orally twice daily 100 X 109/L to 200 X 109/L 15 mg orally twice daily 50 X 109/L to less than 100 X 109/L 5 mg orally twice daily Dose Modification Guidelines for Hematologic Toxicity for Patients with Myelofibrosis Starting Treatment with a Platelet Count of 100 X 109/L or Greater
Treatment Interruption and Restarting Dosing
Interrupt treatment for platelet counts less than 50 X 109/L or absolute neutrophil count (ANC) less than 0.5 X 109/L.
After recovery of platelet counts above 50 X 109/L and ANC above 0.75 X 109/L, dosing may be restarted. Table 2 illustrates the maximum allowable dose that may be used in restarting Jakafi after a previous interruption.
Table 2: Myelofibrosis: Maximum Restarting Doses for Jakafi after Safety Interruption for Thrombocytopenia for Patients Starting Treatment with a Platelet Count of 100 X 109/L or Greater - * Maximum doses are displayed. When restarting, begin with a dose at least 5 mg twice daily below the dose at interruption.
Current Platelet Count Maximum Dose When
Restarting Jakafi Treatment*Greater than or equal to 125 X 109/L 20 mg twice daily 100 to less than 125 X 109/L 15 mg twice daily 75 to less than 100 X 109/L 10 mg twice daily for at least 2 weeks; if stable, may increase to 15 mg twice daily 50 to less than 75 X 109/L 5 mg twice daily for at least 2 weeks; if stable, may increase to 10 mg twice daily Less than 50 X 109/L Continue hold Following treatment interruption for ANC below 0.5 X 109/L, after ANC recovers to 0.75 X 109/L or greater, restart dosing at the higher of 5 mg once daily or 5 mg twice daily below the largest dose in the week prior to the treatment interruption.
Dose Reductions
Dose reductions should be considered if the platelet counts decrease as outlined in Table 3 with the goal of avoiding dose interruptions for thrombocytopenia.Table 3: Myelofibrosis: Dosing Recommendations for Thrombocytopenia for Patients Starting Treatment with a Platelet Count of 100 X 109/L or Greater Dose at Time of Platelet Decline Platelet Count 25 mg
twice
daily20 mg
twice
daily15 mg
twice
daily10 mg
twice
daily5 mg
twice
dailyNew
DoseNew
DoseNew
DoseNew
DoseNew
Dose100 to less than
125 X 109/L20 mg
twice
daily15 mg
twice
dailyNo
ChangeNo
ChangeNo
Change75 to less than
100 X 109/L10 mg
twice
daily10 mg
twice
daily10 mg
twice
dailyNo
ChangeNo
Change50 to less than
75 X 109/L5 mg
twice
daily5 mg
twice
daily5 mg
twice
daily5 mg
twice
dailyNo
ChangeLess than 50 X 109/L Hold Hold Hold Hold Hold Dose Modification Based on Insufficient Response for Patients with Myelofibrosis Starting Treatment with a Platelet Count of 100 X 109/L or Greater
If the response is insufficient and platelet and neutrophil counts are adequate, doses may be increased in 5 mg twice daily increments to a maximum of 25 mg twice daily. Doses should not be increased during the first 4 weeks of therapy and not more frequently than every 2 weeks.
Consider dose increases in patients who meet all of the following conditions:
- Failure to achieve a reduction from pretreatment baseline in either palpable spleen length of 50% or a 35% reduction in spleen volume as measured by computed tomography (CT) or magnetic resonance imaging (MRI);
- Platelet count greater than 125 X 109/L at 4 weeks and platelet count never below 100 X 109/L;
- ANC Levels greater than 0.75 X 109/L.
Based on limited clinical data, long-term maintenance at a 5 mg twice daily dose has not shown responses and continued use at this dose should be limited to patients in whom the benefits outweigh the potential risks. Discontinue Jakafi if there is no spleen size reduction or symptom improvement after 6 months of therapy.
Dose Modifications for Hematologic Toxicity for Patients with Myelofibrosis Starting Treatment with Platelet Counts of 50 X 109/L to Less Than 100 X 109/L
This section applies only to patients with platelet counts of 50 X 109/L to less than 100 X 109/L prior to any treatment with Jakafi. See dose modifications in Section 2.1 (Dose Modification Guidelines for Hematological Toxicity for Patients with Myelofibrosis Starting Treatment with a Platelet Count of 100 X 109/L or Greater) for hematological toxicity in patients whose platelet counts were 100 X 109/L or more prior to starting treatment with Jakafi.
Treatment Interruption and Restarting Dosing
Interrupt treatment for platelet counts less than 25 X 109/L or ANC less than 0.5 X 109/L.
After recovery of platelet counts above 35 X 109/L and ANC above 0.75 X 109/L, dosing may be restarted. Restart dosing at the higher of 5 mg once daily or 5 mg twice daily below the largest dose in the week prior to the decrease in platelet count below 25 X 109/L or ANC below 0.5 X 109/L that led to dose interruption.
Dose Reductions
Reduce the dose of Jakafi for platelet counts less than 35 X 109/L as described in Table 4.
Table 4: Myelofibrosis: Dosing Modifications for Thrombocytopenia for Patients with Starting Platelet Count of 50 X 109/L to Less Than 100 X 109/L Platelet Count Dosing Recommendations Less than 25 X 109/L - Interrupt dosing.
25 X 109/L to less than 35 X 109/L
AND the platelet count decline is
less than 20% during the prior four
weeks- Decrease dose by 5 mg once daily.
- For patients on 5 mg once daily, maintain dose at 5 mg once daily.
25 X 109/L to less than 35 X 109/L
AND the platelet count decline is
20% or greater during the prior four weeks- Decrease dose by 5 mg twice daily.
- For patients on 5 mg twice daily, decrease the dose to 5 mg once daily.
- For patients on 5 mg once daily, maintain dose at 5 mg once daily.
Dose Modifications Based on Insufficient Response for Patients with Myelofibrosis and Starting Platelet Count of 50 X 109/L to Less Than 100 X 109/L
Do not increase doses during the first 4 weeks of therapy, and do not increase the dose more frequently than every 2 weeks.
If the response is insufficient as defined in Section 2.1 (see Dose Modification Based on Insufficient Response with Myelofibrosis Starting Treatment with a platelet count of 100 X 109/L or Greater), doses may be increased by increments of 5 mg daily to a maximum of 10 mg twice daily if:
- the platelet count has remained at least 40 X 109/L, and
- the platelet count has not fallen by more than 20% in the prior 4 weeks, and
- the ANC is more than 1 X 109/L, and
- the dose has not been reduced or interrupted for an adverse event or hematological toxicity in the prior 4 weeks.
Continuation of treatment for more than 6 months should be limited to patients in whom the benefits outweigh the potential risks. Discontinue Jakafi if there is no spleen size reduction or symptom improvement after 6 months of therapy.
Dose Modification for Bleeding
Interrupt treatment for bleeding requiring intervention regardless of current platelet count. Once the bleeding event has resolved, consider resuming treatment at the prior dose if the underlying cause of bleeding has been controlled. If the bleeding event has resolved but the underlying cause persists, consider resuming treatment with Jakafi at a lower dose.
2.2 Polycythemia Vera
The recommended starting dose of Jakafi is 10 mg twice daily. Doses may be titrated based on safety and efficacy.
Dose Modification Guidelines for Patients with Polycythemia Vera
A complete blood count (CBC) and platelet count must be performed before initiating therapy, every 2 to 4 weeks until doses are stabilized, and then as clinically indicated [see Warnings and Precautions (5.1)].
Dose Reductions
Dose reductions should be considered for hemoglobin and platelet count decreases as described in Table 5.
Table 5: Polycythemia Vera: Dose Reductions Hemoglobin and/or Platelet Count Dosing Recommendations Hemoglobin greater than or equal to 12 g/dL AND platelet count greater than or equal to 100 X 109/L - No change required.
Hemoglobin 10 to less than 12 g/dL AND platelet count 75 to less than 100 X 109/L - Dose reductions should be considered with the goal of avoiding dose interruptions for anemia and thrombocytopenia.
Hemoglobin 8 to less than 10 g/dL OR platelet count 50 to less than 75 X 109/L - Reduce dose by 5 mg twice daily.
- For patients on 5 mg twice daily, decrease the dose to 5 mg once daily.
Hemoglobin less than 8 g/dL OR platelet count less than 50 X 109/L - Interrupt dosing.
Treatment Interruption and Restarting Dosing
Interrupt treatment for hemoglobin less than 8 g/dL, platelet counts less than 50 X 109/L or ANC less than 1.0 X 109/L.
After recovery of the hematologic parameter(s) to acceptable levels, dosing may be restarted.
Table 6 illustrates the dose that may be used in restarting Jakafi after a previous interruption.
Table 6: Polycythemia Vera: Restarting Doses for Jakafi after Safety Interruption for Hematologic Parameter(s)
Use the most severe category of a patient’s hemoglobin, platelet count, or ANC abnormality to determine the corresponding maximum restarting dose.
- * Continue treatment for at least 2 weeks; if stable, may increase dose by 5 mg twice daily.
Hemoglobin, Platelet Count, or ANC Maximum Restarting Dose Hemoglobin less than 8 g/dL OR
platelet count less than 50 X 109/L OR
ANC less than 1 X 109/LContinue hold Hemoglobin 8 to less than 10 g/dL OR
platelet count 50 to less than 75 X 109/L OR
ANC 1 to less than 1.5 X 109/L5 mg twice daily*or no more than
5 mg twice daily less than the dose
which resulted in dose interruptionHemoglobin 10 to less than 12 g/dL OR
platelet count 75 to less than 100 X 109/L OR
ANC 1.5 to less than 2 X 109/L10 mg twice daily*or no more than
5 mg twice daily less than the dose
which resulted in dose interruptionHemoglobin greater than or equal to 12 g/dL OR
platelet count greater than or equal to 100 X 109/L OR
ANC greater than or equal to 2 X 109/L15 mg twice daily*or no more than
5 mg twice daily less than the dose
which resulted in dose interruptionPatients who had required dose interruption while receiving a dose of 5 mg twice daily, may restart at a dose of 5 mg twice daily or 5 mg once daily, but not higher, once hemoglobin is greater than or equal to 10 g/dL, platelet count is greater than or equal to 75 X 109/L, and ANC is greater than or equal to 1.5 X 109/L.
Dose Management after Restarting Treatment
After restarting Jakafi following treatment interruption, doses may be titrated, but the maximum total daily dose should not exceed 5 mg less than the dose that resulted in the dose interruption. An exception to this is dose interruption following phlebotomy-associated anemia, in which case the maximal total daily dose allowed after restarting Jakafi would not be limited.
Dose Modifications Based on Insufficient Response for Patients with Polycythemia Vera
If the response is insufficient and platelet, hemoglobin, and neutrophil counts are adequate, doses may be increased in 5 mg twice daily increments to a maximum of 25 mg twice daily. Doses should not be increased during the first 4 weeks of therapy and not more frequently than every two weeks.
Consider dose increases in patients who meet all of the following conditions:
- Inadequate efficacy as demonstrated by one or more of the following:
- Continued need for phlebotomy
- WBC greater than the upper limit of normal range
- Platelet count greater than the upper limit of normal range
- Palpable spleen that is reduced by less than 25% from Baseline
- Platelet count greater than or equal to 140 X 109/L
- Hemoglobin greater than or equal to 12 g/dL
- ANC greater than or equal to 1.5 X 109/L
2.3 Acute Graft-Versus-Host Disease
The recommended starting dose of Jakafi is 5 mg given orally twice daily. Consider increasing the dose to 10 mg twice daily after at least 3 days of treatment if the ANC and platelet counts are not decreased by 50% or more relative to the first day of dosing with Jakafi.
Tapering of Jakafi may be considered after 6 months of treatment in patients with response who have discontinued therapeutic doses of corticosteroids. Taper Jakafi by one dose level approximately every 8 weeks (10 mg twice daily to 5 mg twice daily to 5 mg once daily). If acute GVHD signs or symptoms recur during or after the taper of Jakafi, consider retreatment.
Dose Modification Guidelines for Patients with Acute Graft-Versus-Host Disease
Evaluate blood parameters before and during treatment with Jakafi. Dose reductions should be considered for platelet counts, ANCs or bilirubin value as described in Table 7. Patients who are currently receiving Jakafi 10 mg twice daily may have their dose reduced to 5 mg twice daily; patients receiving 5 mg twice daily may have their dose reduced to 5 mg once daily. Patients who are unable to tolerate Jakafi at a dose of 5 mg once daily should have treatment interrupted until their clinical and/or laboratory parameters recover.
Table 7: Dose Modifications for Patients with Acute GVHD Laboratory Parameter Dosing Recommendations Clinically significant
thrombocytopenia after supportive
measuresReduce dose by 1 dose level.
When platelets recover to previous values, dosing may
return to prior dose level.ANC less than 1 X 109/L
considered related to JakafiHold Jakafi for up to 14 days; resume at 1 dose level
lower upon recovery.Total Bilirubin elevation, no liver
GVHD3.0−5.0 × ULN: Continue Jakafi at 1 dose level lower
until recovery.>5.0−10.0 × ULN: Hold Jakafi for up to 14 days until
bilirubin ≤ 1.5 × ULN; resume at current dose upon
recoveryTotal bilirubin > 10.0 × ULN: Hold Jakafi for up to
14 days until bilirubin ≤ 1.5 × ULN; resume at 1 dose
level lower upon recovery.Total Bilirubin elevation, liver
GVHD>3.0 × ULN: Continue Jakafi at 1 dose level lower
until recovery.
2.4 Dose Modifications for Concomitant Use with Strong CYP3A4 Inhibitors or Fluconazole
Modify the Jakafi dosage when coadministered with strong CYP3A4 inhibitors and fluconazole doses of less than or equal to 200 mg [see Drug Interactions (7)], according to Table 8.
Additional dose modifications should be made with frequent monitoring of safety and efficacy.
Avoid the use of fluconazole doses of greater than 200 mg daily with Jakafi except in patients with acute GVHD.
Table 8: Dose Modifications for Concomitant Use with Strong CYP3A4 Inhibitors or Fluconazole - *
With coadministration of itraconazole, monitor blood counts more frequently for toxicity and adjust the dose of Jakafi if necessary.
For patients coadministered strong CYP3A4 inhibitors or fluconazole doses of less than or equal to 200 mg Recommended Dose Modification Starting dose for patients with MF with a platelet count: - Greater than or equal to 100 X 109/L
10 mg twice daily - 50 X 109/L to less than 100 X 109/L
5 mg once daily Starting dose for patients with PV: 5 mg twice daily If on stable dose for patients with MF or PV: - Greater than or equal to 10 mg twice daily
Decrease dose by 50%
(round up to the closest available tablet strength)- 5 mg twice daily
5 mg once daily - 5 mg once daily
Avoid strong CYP3A4 inhibitor or fluconazole treatment or interrupt Jakafi treatment for the duration of strong CYP3A4 inhibitor or fluconazole use For patients with acute GVHD: Ketoconazole 5 mg once daily Other CYP3A4 inhibitors* No dose adjustment 2.5 Dose Modifications for Renal or Hepatic Impairment
Renal Impairment
Patients with Moderate or Severe Renal Impairment
Modify the Jakafi dosage for patients with moderate or severe renal impairment according to Table 9.
Patients with End Stage Renal Disease on Dialysis
Modify the Jakafi dosage for patients with end stage renal disease (ESRD) on dialysis according to Table 9. Make additional dose modifications with frequent monitoring of safety and efficacy. Avoid use of Jakafi in patients with ESRD (CLcr less than 15 mL/min) not requiring dialysis [see Use in Specific Populations (8.6)].
Table 9: Dose Modifications for Renal Impairment Renal Impairment Status Platelet Count Recommended Starting Dosage Patients with MF
Moderate (CLcr 30 to 59 mL/min) or
Severe (CLcr 15 to 29 mL/min)Greater than 150 X 109/L No dose modification needed 100 to 150 X 109/L 10 mg twice daily 50 to less than 100 X 109/L 5 mg daily Less than 50 X 109/L Avoid use [see Use in Specific
Populations (8.6)]ESRD (CLcr less than 15 mL/min) on
dialysis100 to 200 X 109/L 15 mg once after dialysis session Greater than 200 X 109/L
20 mg once after dialysis session Patients with PV Moderate (CLcr 30 to 59 mL/min) or
Severe (CLcr 15 to 29 mL/min)Any 5 mg twice daily ESRD (CLcr less than 15 mL/min) on
dialysisAny 10 mg once after dialysis session Patients with acute GVHD Moderate (CLcr 30 to 59 mL/min) or
Severe (CLcr 15 to 29 mL/min)Any 5 mg once daily ESRD (CLcr less than 15 mL/min) on
dialysisAny 5 mg once after dialysis session ESRD = end stage renal disease, and CLcr = creatinine clearance
Hepatic Impairment
Modify the Jakafi dosage for patients with hepatic impairment according to Table 10.
Table 10: Dose Modifications for Hepatic Impairment Hepatic Impairment Status Platelet Count Recommended Starting Dosage Patients with MF
Mild, Moderate, or Severe (Child-
Pugh Class A, B, C)Greater than 150 X 109/L No dose modification needed 100 X 109/L to 150 X 109/L 10 mg twice daily 50 to less than 100 X 109/L 5 mg daily Less than 50 X 109/L Avoid use [see Use in Specific
Populations (8.7)]Patients with PV
Mild, Moderate, or Severe (Child-
Pugh Class A, B, C)Any 5 mg twice daily Patients with acute GVHD
Mild, Moderate, or Severe based
on NCI criteriaAny No dose modification needed
Stage 3 or 4 liver GVHDAny Monitor blood counts more
frequently for toxicity and
consider 5 mg once daily2.6 Method of Administration
Jakafi is dosed orally and can be administered with or without food.
If a dose is missed, the patient should not take an additional dose, but should take the next usual prescribed dose.
When discontinuing Jakafi therapy for reasons other than thrombocytopenia, gradual tapering of the dose of Jakafi may be considered, for example by 5 mg twice daily each week.
For patients unable to ingest tablets, Jakafi can be administered through a nasogastric tube (8 French or greater) as follows:
- Suspend one tablet in approximately 40 mL of water with stirring for approximately 10 minutes.
- Within 6 hours after the tablet has dispersed, the suspension can be administered through a nasogastric tube using an appropriate syringe.
The tube should be rinsed with approximately 75 mL of water. The effect of tube feeding preparations on Jakafi exposure during administration through a nasogastric tube has not been evaluated.
-
3. DOSAGE FORMS AND STRENGTHS
5 mg tablets - round and white with "INCY" on one side and "5" on the other.
10 mg tablets - round and white with "INCY" on one side and "10" on the other.
15 mg tablets - oval and white with "INCY" on one side and "15" on the other.
20 mg tablets - capsule-shaped and white with "INCY" on one side and "20" on the other.
25 mg tablets - oval and white with "INCY" on one side and "25" on the other.
- 4. CONTRAINDICATIONS
-
5. WARNINGS AND PRECAUTIONS
5.1 Thrombocytopenia, Anemia and Neutropenia
Treatment with Jakafi can cause thrombocytopenia, anemia and neutropenia [see Dosage and Administration (2.1)].
Manage thrombocytopenia by reducing the dose or temporarily interrupting Jakafi. Platelet transfusions may be necessary [see Dosage and Administration (2), and Adverse Reactions (6.1)].
Patients developing anemia may require blood transfusions and/or dose modifications of Jakafi.
Severe neutropenia (ANC less than 0.5 X 109/L) was generally reversible by withholding Jakafi until recovery [see Adverse Reactions (6.1)].
Perform a pre-treatment complete blood count (CBC) and monitor CBCs every 2 to 4 weeks until doses are stabilized, and then as clinically indicated [see Dosage and Administration (2), and Adverse Reactions (6.1)].
5.2 Risk of Infection
Serious bacterial, mycobacterial, fungal and viral infections have occurred. Delay starting therapy with Jakafi until active serious infections have resolved. Observe patients receiving Jakafi for signs and symptoms of infection and manage promptly. Use active surveillance and prophylactic antibiotics according to clinical guidelines.
Tuberculosis
Tuberculosis infection has been reported in patients receiving Jakafi. Observe patients receiving Jakafi for signs and symptoms of active tuberculosis and manage promptly.
Prior to initiating Jakafi, patients should be evaluated for tuberculosis risk factors, and those at higher risk should be tested for latent infection. Risk factors include, but are not limited to, prior residence in or travel to countries with a high prevalence of tuberculosis, close contact with a person with active tuberculosis, and a history of active or latent tuberculosis where an adequate course of treatment cannot be confirmed.
For patients with evidence of active or latent tuberculosis, consult a physician with expertise in the treatment of tuberculosis before starting Jakafi. The decision to continue Jakafi during treatment of active tuberculosis should be based on the overall risk-benefit determination.
Progressive Multifocal Leukoencephalopathy
Progressive multifocal leukoencephalopathy (PML) has occurred with Jakafi treatment. If PML is suspected, stop Jakafi and evaluate.
Herpes Zoster
Advise patients about early signs and symptoms of herpes zoster and to seek treatment as early as possible if suspected [see Adverse Reactions (6.1)].
Hepatitis B
Hepatitis B viral load (HBV-DNA titer) increases, with or without associated elevations in alanine aminotransferase and aspartate aminotransferase, have been reported in patients with chronic HBV infections taking Jakafi. The effect of Jakafi on viral replication in patients with chronic HBV infection is unknown. Patients with chronic HBV infection should be treated and monitored according to clinical guidelines.
5.3 Symptom Exacerbation Following Interruption or Discontinuation of Treatment with Jakafi
Following discontinuation of Jakafi, symptoms from myeloproliferative neoplasms may return to pretreatment levels over a period of approximately one week. Some patients with MF have experienced one or more of the following adverse events after discontinuing Jakafi: fever, respiratory distress, hypotension, DIC, or multi-organ failure. If one or more of these occur after discontinuation of, or while tapering the dose of Jakafi, evaluate for and treat any intercurrent illness and consider restarting or increasing the dose of Jakafi. Instruct patients not to interrupt or discontinue Jakafi therapy without consulting their physician. When discontinuing or interrupting therapy with Jakafi for reasons other than thrombocytopenia or neutropenia [see Dosage and Administration (2.6)], consider tapering the dose of Jakafi gradually rather than discontinuing abruptly.
5.4 Non-Melanoma Skin Cancer
Non-melanoma skin cancers including basal cell, squamous cell, and Merkel cell carcinoma have occurred in patients treated with Jakafi. Perform periodic skin examinations.
5.5 Lipid Elevations
Treatment with Jakafi has been associated with increases in lipid parameters including total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides. The effect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined in patients treated with Jakafi. Assess lipid parameters approximately 8-12 weeks following initiation of Jakafi therapy. Monitor and treat according to clinical guidelines for the management of hyperlipidemia.
-
6. ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Thrombocytopenia, Anemia and Neutropenia [see Warnings and Precautions (5.1)]
- Risk of Infection [see Warnings and Precautions (5.2)]
- Symptom Exacerbation Following Interruption or Discontinuation of Treatment with Jakafi [see Warnings and Precautions (5.3)]
- Non-Melanoma Skin Cancer [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience in Myelofibrosis
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of Jakafi was assessed in 617 patients in six clinical studies with a median duration of follow-up of 10.9 months, including 301 patients with MF in two Phase 3 studies.
In these two Phase 3 studies, patients had a median duration of exposure to Jakafi of 9.5 months (range 0.5 to 17 months), with 89% of patients treated for more than 6 months and 25% treated for more than 12 months. One hundred and eleven (111) patients started treatment at 15 mg twice daily and 190 patients started at 20 mg twice daily. In patients starting treatment with 15 mg twice daily (pretreatment platelet counts of 100 to 200 X 109/L) and 20 mg twice daily (pretreatment platelet counts greater than 200 X 109/L), 65% and 25% of patients, respectively, required a dose reduction below the starting dose within the first 8 weeks of therapy.
In a double-blind, randomized, placebo-controlled study of Jakafi, among the 155 patients treated with Jakafi, the most frequent adverse reactions were thrombocytopenia and anemia [see Table 12]. Thrombocytopenia, anemia and neutropenia are dose-related effects. The three most frequent nonhematologic adverse reactions were bruising, dizziness and headache [see Table 11].
Discontinuation for adverse events, regardless of causality, was observed in 11% of patients treated with Jakafi and 11% of patients treated with placebo.
Table 11 presents the most common nonhematologic adverse reactions occurring in patients who received Jakafi in the double-blind, placebo-controlled study during randomized treatment.
Table 11: Myelofibrosis: Nonhematologic Adverse Reactions Occurring in Patients on Jakafi in the Double-blind, Placebo-controlled Study During Randomized Treatment - * National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 3.0
- † includes contusion, ecchymosis, hematoma, injection site hematoma, periorbital hematoma, vessel puncture site hematoma, increased tendency to bruise, petechiae, purpura
- ‡ includes dizziness, postural dizziness, vertigo, balance disorder, Meniere's Disease, labyrinthitis
- § includes urinary tract infection, cystitis, urosepsis, urinary tract infection bacterial, kidney infection, pyuria, bacteria urine, bacteria urine identified, nitrite urine present
- ¶ includes weight increased, abnormal weight gain
- # includes herpes zoster and post-herpetic neuralgia
Jakafi
(N=155)Placebo
(N=151)Adverse Reactions All
Grades*
(%)Grade 3
(%)Grade 4
(%)All
Grades
(%)Grade 3
(%)Grade 4
(%)Bruising† 23 <1 0 15 0 0 Dizziness‡ 18 <1 0 7 0 0 Headache 15 0 0 5 0 0 Urinary Tract Infections§ 9 0 0 5 <1 <1 Weight Gain¶ 7 <1 0 1 <1 0 Flatulence 5 0 0 <1 0 0 Herpes Zoster# 2 0 0 <1 0 0 Description of Selected Adverse Reactions
Anemia
In the two Phase 3 clinical studies, median time to onset of first CTCAE Grade 2 or higher anemia was approximately 6 weeks. One patient (<1%) discontinued treatment because of anemia. In patients receiving Jakafi, mean decreases in hemoglobin reached a nadir of approximately 1.5 to 2.0 g/dL below baseline after 8 to 12 weeks of therapy and then gradually recovered to reach a new steady state that was approximately 1.0 g/dL below baseline. This pattern was observed in patients regardless of whether they had received transfusions during therapy.
In the randomized, placebo-controlled study, 60% of patients treated with Jakafi and 38% of patients receiving placebo received red blood cell transfusions during randomized treatment. Among transfused patients, the median number of units transfused per month was 1.2 in patients treated with Jakafi and 1.7 in placebo treated patients.
Thrombocytopenia
In the two Phase 3 clinical studies, in patients who developed Grade 3 or 4 thrombocytopenia, the median time to onset was approximately 8 weeks. Thrombocytopenia was generally reversible with dose reduction or dose interruption. The median time to recovery of platelet counts above 50 X 109/L was 14 days. Platelet transfusions were administered to 5% of patients receiving Jakafi and to 4% of patients receiving control regimens. Discontinuation of treatment because of thrombocytopenia occurred in <1% of patients receiving Jakafi and <1% of patients receiving control regimens. Patients with a platelet count of 100 X 109/L to 200 X 109/L before starting Jakafi had a higher frequency of Grade 3 or 4 thrombocytopenia compared to patients with a platelet count greater than 200 X 109/L (17% versus 7%).
Neutropenia
In the two Phase 3 clinical studies, 1% of patients reduced or stopped Jakafi because of neutropenia.
Table 12 provides the frequency and severity of clinical hematology abnormalities reported for patients receiving treatment with Jakafi or placebo in the placebo-controlled study.
Table 12: Myelofibrosis: Worst Hematology Laboratory Abnormalities in the Placebo-Controlled Study* - *
Presented values are worst Grade values regardless of baseline
- †
National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0
Jakafi
(N=155)Placebo
(N=151)Laboratory Parameter
All
Grades†
(%)Grade 3
(%)Grade 4
(%)All
Grades
(%)Grade 3
(%)Grade 4
(%)Thrombocytopenia 70 9 4 31 1 0 Anemia 96 34 11 87 16 3 Neutropenia 19 5 2 4 <1 1 Additional Data from the Placebo-Controlled Study
- 25% of patients treated with Jakafi and 7% of patients treated with placebo developed newly occurring or worsening Grade 1 abnormalities in alanine transaminase (ALT). The incidence of greater than or equal to Grade 2 elevations was 2% for Jakafi with 1% Grade 3 and no Grade 4 ALT elevations.
- 17% of patients treated with Jakafi and 6% of patients treated with placebo developed newly occurring or worsening Grade 1 abnormalities in aspartate transaminase (AST). The incidence of Grade 2 AST elevations was <1% for Jakafi with no Grade 3 or 4 AST elevations.
- 17% of patients treated with Jakafi and <1% of patients treated with placebo developed newly occurring or worsening Grade 1 elevations in cholesterol. The incidence of Grade 2 cholesterol elevations was <1% for Jakafi with no Grade 3 or 4 cholesterol elevations.
6.2 Clinical Trial Experience in Polycythemia Vera
In a randomized, open-label, active-controlled study, 110 patients with PV resistant to or intolerant of hydroxyurea received Jakafi and 111 patients received best available therapy [see Clinical Studies (14.2)]. The most frequent adverse reaction was anemia. Discontinuation for adverse events, regardless of causality, was observed in 4% of patients treated with Jakafi. Table 13 presents the most frequent nonhematologic adverse reactions occurring up to Week 32.
Table 13: Polycythemia Vera: Nonhematologic Adverse Reactions Occurring in ≥ 5% of Patients on Jakafi in the Open-Label, Active-controlled Study up to Week 32 of Randomized Treatment - * National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 3.0
- † includes dizziness and vertigo
- ‡ includes dyspnea and dyspnea exertional
- § includes herpes zoster and post-herpetic neuralgia
- ¶
includes weight increased and abnormal weight gain
- #
includes urinary tract infection and cystitis
Jakafi
(N=110)Best Available Therapy
(N=111)Adverse Reactions
All Grades*
(%)Grade 3-4
(%)All Grades
(%)Grade 3-4
(%)Diarrhea 15 0 7 <1 Dizziness† 15 0 13 0 Dyspnea‡ 13 3 4 0 Muscle Spasms 12 <1 5 0 Constipation 8 0 3 0 Herpes Zoster§ 6 <1 0 0 Nausea 6 0 4 0 Weight Gain¶ 6 0 <1 0 Urinary Tract Infections# 6 0 3 0 Hypertension 5 <1 3 <1 Clinically relevant laboratory abnormalities are shown in Table 14.
Table 14: Polycythemia Vera: Selected Laboratory Abnormalities in the Open-Label, Active-controlled Study up to Week 32 of Randomized Treatment* - *
Presented values are worst Grade values regardless of baseline
- †
National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0
Jakafi
(N=110)Best Available Therapy
(N=111)Laboratory Parameter All
Grades†
(%)Grade 3
(%)Grade 4
(%)All
Grades
(%)Grade 3
(%)Grade 4
(%)Hematology Anemia 72 <1 <1 58 0 0 Thrombocytopenia 27 5 <1 24 3 <1 Neutropenia 3 0 <1 10 <1 0 Chemistry Hypercholesterolemia 35 0 0 8 0 0 Elevated ALT 25 <1 0 16 0 0 Elevated AST 23 0 0 23 <1 0 Hypertriglyceridemia 15 0 0 13 0 0 6.3 Clinical Trial Experience in Acute Graft-Versus-Host Disease
In a single-arm, open-label study, 71 adults (ages 18-73 years) were treated with Jakafi for acute GVHD failing treatment with steroids with or without other immunosuppressive drugs [see Clinical Studies(14.3)]. The median duration of treatment with Jakafi was 46 days (range, 4‑382 days).
There were no fatal adverse reactions to Jakafi. An adverse reaction resulting in treatment discontinuation occurred in 31% of patients. The most common adverse reaction leading to treatment discontinuation was infection (10%). Table 15 shows the adverse reactions other than laboratory abnormalities.
Table 15: Acute Graft-Versus-Host Disease: Nonhematologic Adverse Reactions Occurring in ≥ 15% of Patients in the Open-Label, Single-Cohort Study - * Selected laboratory abnormalities are listed in Table 16 below
- † National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.03
Jakafi (N=71) Adverse Reactions* All Grades†
(%)Grade 3-4
(%)Infections 55
41 Edema 51 13 Hemorrhage 49 20 Fatigue 37 14 Bacterial infections 32 28 Dyspnea 32 7 Viral infections 31 14 Thrombosis 25 11 Diarrhea 24 7 Rash 23 3 Headache 21 4 Hypertension 20 13 Dizziness 16 0 Selected laboratory abnormalities during treatment with Jakafi are shown in Table 16.
Table 16: Acute Graft-Versus-Host Disease: Selected Laboratory Abnormalities Worsening from Baseline in the Open-Label, Single Cohort Study - * National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03
Jakafi (N=71) Worst grade during treatment Laboratory Parameter All Grades*
(%)Grade 3-4
(%)Hematology Anemia 75 45 Thrombocytopenia 75 61 Neutropenia 58 40 Chemistry Elevated ALT 48 8 Elevated AST 48 6 Hypertriglyceridemia 11 1 -
7. DRUG INTERACTIONS
Fluconazole
Concomitant administration of Jakafi with fluconazole doses greater than 200 mg daily may increase ruxolitinib exposure due to inhibition of both the CYP3A4 and CYP2C9 metabolic pathways [see Clinical Pharmacology (12.3)]. Increased exposure may increase the risk of exposure-related adverse reactions. Avoid the concomitant use of Jakafi with fluconazole doses of greater than 200 mg daily except in patients with acute GVHD [see Dosage and Administration (2.4)].
Strong CYP3A4 inhibitors
Concomitant administration of Jakafi with strong CYP3A4 inhibitors increases ruxolitinib exposure [see Clinical Pharmacology (12.3)]. Increased exposure may increase the risk of exposure-related adverse reactions. Consider dose reduction when administering Jakafi with strong CYP3A4 inhibitors [see Dosage and Administration (2.4)]. In patients with acute GVHD, reduce Jakafi dose as recommended only when coadministered with ketoconazole, and monitor blood counts more frequently for toxicity and adjust the dose if necessary when coadministered with itraconazole [see Dosage and Administration (2.4)].
Strong CYP3A4 inducers
Concomitant administration of Jakafi with strong CYP3A4 inducers may decrease ruxolitinib exposure [see Clinical Pharmacology (12.3)]. No dose adjustment is recommended; however, monitor patients frequently and adjust the Jakafi dose based on safety and efficacy [see Clinical Pharmacology (12.3)].
-
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
When pregnant rats and rabbits were administered ruxolitinib during the period of organogenesis adverse developmental outcomes occurred at doses associated with maternal toxicity (see Data). There are no studies with the use of Jakafi in pregnant women to inform drug-associated risks.
The background risk of major birth defects and miscarriage for the indicated populations is unknown. Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. The background risk in the U.S. general population of major birth defects is 2% to 4% and miscarriage is 15% to 20% of clinically recognized pregnancies.
Data
Animal Data
Ruxolitinib was administered orally to pregnant rats or rabbits during the period of organogenesis, at doses of 15, 30 or 60 mg/kg/day in rats and 10, 30 or 60 mg/kg/day in rabbits. There were no treatment-related malformations. Adverse developmental outcomes, such as decreases of approximately 9% in fetal weights were noted in rats at the highest and maternally toxic dose of 60 mg/kg/day. This dose results in an exposure (AUC) that is approximately 2 times the clinical exposure at the maximum recommended dose of 25 mg twice daily. In rabbits, lower fetal weights of approximately 8% and increased late resorptions were noted at the highest and maternally toxic dose of 60 mg/kg/day. This dose is approximately 7% the clinical exposure at the maximum recommended dose.
In a pre- and post-natal development study in rats, pregnant animals were dosed with ruxolitinib from implantation through lactation at doses up to 30 mg/kg/day. There were no drug-related adverse findings in pups for fertility indices or for maternal or embryofetal survival, growth and development parameters at the highest dose evaluated (34% the clinical exposure at the maximum recommended dose of 25 mg twice daily).
8.2 Lactation
Risk Summary
No data are available regarding the presence of ruxolitinib in human milk, the effects on the breast fed child, or the effects on milk production. Ruxolitinib and/or its metabolites were present in the milk of lactating rats (see Data). Because many drugs are present in human milk and because of the potential for thrombocytopenia and anemia shown for Jakafi in human studies, discontinue breastfeeding during treatment with Jakafi and for two weeks after the final dose.
Data
Animal Data
Lactating rats were administered a single dose of [14C]-labeled ruxolitinib (30 mg/kg) on postnatal Day 10, after which plasma and milk samples were collected for up to 24 hours. The AUC for total radioactivity in milk was approximately 13-fold the maternal plasma AUC. Additional analysis showed the presence of ruxolitinib and several of its metabolites in milk, all at levels higher than those in maternal plasma.
8.4 Pediatric Use
The safety and effectiveness of Jakafi for treatment of myelofibrosis or polycythemia vera in pediatric patients have not been established.
The safety and effectiveness of Jakafi for treatment of steroid-refractory acute graft-versus-host disease (GVHD) have been established for treatment of children 12 years and older. Use of Jakafi in pediatric patients with steroid-refractory acute GVHD is supported by evidence from an adequate and well-controlled trial of Jakafi in adults [see Clinical Studies (14.3)] and additional pharmacokinetic and safety data in pediatric patients.
Jakafi was evaluated in a single-arm, dose-escalation study (NCT01164163) in 27 pediatric patients with relapsed or refractory solid tumors (Cohort A) and 20 with leukemias or myeloproliferative neoplasms (Cohort B). The patients had a median age of 14 years (range, 2 to 21 years) and included 18 children (age 2 to <12 years), and 14 adolescents (age 12 to <17 years). The dose levels tested were 15, 21, 29, 39, or 50 mg/m2 twice daily in 28-day cycles with up to 6 patients per dose group.
Overall, 38 (81%) patients were treated with no more than a single cycle of Jakafi, while 3, 1, 2, and 3 patients received 2, 3, 4, and 5 or more cycles, respectively. A protocol-defined maximal tolerated dose was not observed, but since few patients were treated for multiple cycles, tolerability with continued use was not assessed adequately to establish a recommended Phase 2 dose higher than the recommended dose for adults. The safety profile in children was similar to that seen in adults.
Juvenile Animal Toxicity Data
Administration of ruxolitinib to juvenile rats resulted in effects on growth and bone measures. When administered starting at postnatal day 7 (the equivalent of a human newborn) at doses of 1.5 to 75 mg/kg/day, evidence of fractures occurred at doses ≥ 30 mg/kg/day, and effects on body weight and other bone measures [e.g., bone mineral content, peripheral quantitative computed tomography, and x-ray analysis] occurred at doses ≥ 5 mg/kg/day. When administered starting at postnatal day 21 (the equivalent of a human 2-3 years of age) at doses of 5 to 60 mg/kg/day, effects on body weight and bone occurred at doses ≥ 15 mg/kg/day, which were considered adverse at 60 mg/kg/day. Males were more severely affected than females in all age groups, and effects were generally more severe when administration was initiated earlier in the postnatal period. These findings were observed at exposures that are at least 27% the clinical exposure at the maximum recommended dose of 25 mg twice daily.
8.5 Geriatric Use
Of the total number of patients with MF in clinical studies with Jakafi, 52% were 65 years and older, while 15% were 75 years and older. No overall differences in safety or effectiveness of Jakafi were observed between these patients and younger patients.
Clinical studies of Jakafi in patients with acute GVHD did not include sufficient numbers of subjects age 65 and over to determine whether they respond differently from younger subjects.
8.6 Renal Impairment
Total exposure of ruxolitinib and its active metabolites increased with moderate (CLcr 30 mL/min to 59 mL/min) and severe (CLcr 15 mL/min to 29 mL/min) renal impairment, and ESRD on dialysis [see Clinical Pharmacology (12.3)]. Reduce Jakafi dose as recommended [see Dosage and Administration (2.5)].
8.7 Hepatic Impairment
Exposure of ruxolitinib increased with mild (Child-Pugh A), moderate (Child-Pugh B) and severe (Child-Pugh C) hepatic impairment [see Clinical Pharmacology (12.3)].
Reduce Jakafi dose as recommended in patients with MF or PV and any hepatic impairment [see Dosage and Administration (2.5)].Monitor blood counts more frequently for toxicity and consider 5 mg once daily for patients with Stage 3 or 4 liver GVHD [see Dosage and Administration (2.5) and Clinical Pharmacology (12.3)].
-
10. OVERDOSAGE
There is no known antidote for overdoses with Jakafi. Single doses up to 200 mg have been given with acceptable acute tolerability. Higher than recommended repeat doses are associated with increased myelosuppression including leukopenia, anemia and thrombocytopenia. Appropriate supportive treatment should be given.
Hemodialysis is not expected to enhance the elimination of Jakafi.
-
11. DESCRIPTION
Ruxolitinib phosphate is a kinase inhibitor with the chemical name (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile phosphate and a molecular weight of 404.36. Ruxolitinib phosphate has the following structural formula:
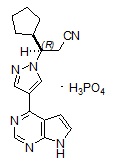
Ruxolitinib phosphate is a white to off-white to light pink powder and is soluble in aqueous buffers across a pH range of 1 to 8.
Jakafi (ruxolitinib) Tablets are for oral administration. Each tablet contains ruxolitinib phosphate equivalent to 5 mg, 10 mg, 15 mg, 20 mg and 25 mg of ruxolitinib free base together with microcrystalline cellulose, lactose monohydrate, magnesium stearate, colloidal silicon dioxide, sodium starch glycolate, povidone and hydroxypropyl cellulose.
-
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ruxolitinib, a kinase inhibitor, inhibits Janus Associated Kinases (JAKs) JAK1 and JAK2 which mediate the signaling of a number of cytokines and growth factors that are important for hematopoiesis and immune function. JAK signaling involves recruitment of STATs (signal transducers and activators of transcription) to cytokine receptors, activation and subsequent localization of STATs to the nucleus leading to modulation of gene expression.
MF and PV are myeloproliferative neoplasms (MPN) known to be associated with dysregulated JAK1 and JAK2 signaling. In a mouse model of JAK2V617F-positive MPN, oral administration of ruxolitinib prevented splenomegaly, preferentially decreased JAK2V617F mutant cells in the spleen and decreased circulating inflammatory cytokines (e.g., TNF-α, IL-6).
JAK-STAT signaling pathways play a role in regulating the development, proliferation, and activation of several immune cell types important for GVHD pathogenesis. In a mouse model of acute GVHD, oral administration of ruxolitinib was associated with decreased expression of inflammatory cytokines in colon homogenates and reduced immune-cell infiltration in the colon.
12.2 Pharmacodynamics
Jakafi inhibits cytokine induced STAT3 phosphorylation in whole blood from patients with MF and PV. Jakafi administration resulted in maximal inhibition of STAT3 phosphorylation 2 hours after dosing which returned to near baseline by 10 hours in patients with MF and PV.
Cardiac Electrophysiology
At a dose of 1.25 to 10 times the highest recommended starting dosage, Jakafi does not prolong the QT interval to any clinically relevant extent.
12.3 Pharmacokinetics
Mean ruxolitinib maximal plasma concentration (Cmax) and AUC increased proportionally over a single dose range of 5 mg to 200 mg. Mean ruxolitinib Cmax ranged from 205 nM to 7100 nM and AUC ranged from 862 nM*hr to 30700 nM*hr over a single dose range of 5 mg to 200 mg.
Absorption
Ruxolitinib achieves Cmax within 1 hour to 2 hours post-dose. Oral absorption of ruxolitinib is estimated to be at least 95%.
Food Effect
No clinically relevant changes in the pharmacokinetics of ruxolitinib were observed upon administration of Jakafi with a high-fat, high-calorie meal (approximately 800 to 1000 calories of which 50% were derived from fat).
Distribution
The mean volume of distribution at steady-state is 72 L (coefficient of variation [CV] 29%) in patients with MF and 75 L (23%) in patients with PV.
Binding to plasma proteins is approximately 97%, mostly to albumin.
Elimination
The mean elimination half-life of ruxolitinib is approximately 3 hours and the mean half-life of ruxolitinib + metabolites is approximately 5.8 hours.
Ruxolitinib clearance (% coefficient of variation, CV) was 17.7 L/h in women and 22.1 L/h in men with MF (39%).
Ruxolitinib clearance (%CV) was 12.7 L/h (42%) in patients with PV.
Ruxolitinib clearance (%CV) was 11.9 L/h (43%) in patients with acute GVHD.
Metabolism
Ruxolitinib is metabolized by CYP3A4 and to a lesser extent by CYP2C9.
Excretion
Following a single oral dose of radiolabeled ruxolitinib, elimination was predominately through metabolism with 74% of radioactivity excreted in urine and 22% excretion via feces. Unchanged drug accounted for less than 1% of the excreted total radioactivity.
Specific Populations
No clinically relevant differences in ruxolitinib pharmacokinetics were observed with regard to age, race, sex, or weight. No clinically relevant effect in ruxolitinib pharmacokinetics were observed with regards to any hepatic impairment (total bilirubin >ULN and any aspartate transferase) in patients with acute GVHD.
Patients with Renal Impairment
Following oral administration of a single dose of Jakafi 25 mg, the total AUC of ruxolitinib and its active metabolites increased by 1.3-,1.5-, and 1.9-fold in subjects with mild, moderate, and severe renal impairment, respectively, compared to that in subjects with normal renal function (CLcr ≥ 90 mL/min). Also, the total AUC of ruxolitinib and its active metabolites increased by 1.6-fold in subjects with ESRD after dialysis) compared to that in subjects with normal renal function (CLcr ≥ 90 mL/min). The pharmacokinetics of ruxolitinib was similar in subjects with various degrees of renal impairment and in those with normal renal function. The change in the pharmacodynamic marker, pSTAT3 inhibition, was consistent with the corresponding increase in metabolite exposure with renal impairment. Ruxolitinib is not removed by dialysis; however, the removal of some active metabolites by dialysis cannot be ruled out.
Patients with Hepatic Impairment
Following oral administration of a single dose of Jakafi 25 mg, the AUC of ruxolitinib increased in subjects with mild (Child-Pugh A) by 1.9-fold, moderate (Child-Pugh B) by 1.3-fold, and severe (Child-Pugh C) hepatic impairment by 1.7-fold compared to that in subjects with normal hepatic function.
The change in the pharmacodynamic marker, pSTAT3 inhibition, was consistent with the corresponding increase in ruxolitinib exposure except in the severe hepatic impairment cohort where the pharmacodynamic activity was more prolonged in some subjects than expected based on plasma concentrations of ruxolitinib.
Drug Interactions
Fluconazole
Simulations suggest that fluconazole (a dual CYP3A4 and CYP2C9 inhibitor) increases steady state ruxolitinib AUC by approximately 100% to 300% following concomitant administration of 10 mg of Jakafi twice daily with 100 mg to 400 mg of fluconazole once daily [see Dosage and Administration (2.4) and Drug Interactions (7)].
Strong CYP3A4 inhibitors
Ketoconazole (a strong CYP3A4 inhibitor) increased ruxolitinib Cmax by 33% and AUC by 91%. Ketoconazole also prolonged ruxolitinib half-life from 3.7 hours to 6 hours [see Dosage and Administration (2.4) and Drug Interactions (7)].
Moderate CYP3A4 inhibitors
Erythromycin (a moderate CYP3A4 inhibitor) increased ruxolitinib Cmax by 8% and AUC by 27% [see Drug Interactions (7)].
Strong CYP3A4 inducers
Rifampin (a strong CYP3A4 inducer) decreased ruxolitinib Cmax by 32% and AUC by 61%. The relative exposure to ruxolitinib’s active metabolites increased approximately 100% [see Drug Interactions (7)].
In vitro studies
Ruxolitinib and its M18 metabolite did not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or CYP3A4. Ruxolitinib did not induce CYP1A2, CYP2B6 or CYP3A4 at clinically relevant concentrations.
Ruxolitinib and its M18 metabolite did not inhibit the P-gp, BCRP, OATP1B1, OATP1B3, OCT1, OCT2, OAT1 or OAT3 transport systems at clinically relevant concentrations. Ruxolitinib is not a substrate for the P-gp transporter.
-
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Ruxolitinib was not carcinogenic in the 6-month Tg.rasH2 transgenic mouse model or in a 2-year carcinogenicity study in the rat.
Ruxolitinib was not mutagenic in a bacterial mutagenicity assay (Ames test) or clastogenic in in vitro chromosomal aberration assay (cultured human peripheral blood lymphocytes) or in vivo in a rat bone marrow micronucleus assay.
In a fertility study, ruxolitinib was administered to male rats prior to and throughout mating and to female rats prior to mating and up to the implantation day (gestation day 7). Ruxolitinib had no effect on fertility or reproductive function in male or female rats at doses of 10, 30 or 60 mg/kg/day. However, in female rats doses of greater than or equal to 30 mg/kg/day resulted in increased post-implantation loss. The exposure (AUC) at the dose of 30 mg/kg/day is approximately 34% the clinical exposure at the maximum recommended dose of 25 mg twice daily.
-
14. CLINICAL STUDIES
14.1 Myelofibrosis
Two randomized Phase 3 studies (Studies 1 and 2) were conducted in patients with MF (either primary MF, post-polycythemia vera MF or post-essential thrombocythemia-MF). In both studies, patients had palpable splenomegaly at least 5 cm below the costal margin and risk category of intermediate 2 (2 prognostic factors) or high risk (3 or more prognostic factors) based on the International Working Group Consensus Criteria (IWG).
The starting dose of Jakafi was based on platelet count. Patients with a platelet count between 100 and 200 X 109/L were started on Jakafi 15 mg twice daily and patients with a platelet count greater than 200 X 109/L were started on Jakafi 20 mg twice daily. Doses were then individualized based upon tolerability and efficacy with maximum doses of 20 mg twice daily for patients with platelet counts between 100 to less than or equal to 125 X 109/L, of 10 mg twice daily for patients with platelet counts between 75 to less than or equal to 100 X 109/L, and of 5 mg twice daily for patients with platelet counts between 50 to less than or equal to 75 X 109/L.
Study 1
Study 1 (NCT00952289) was a double-blind, randomized, placebo-controlled study in 309 patients who were refractory to or were not candidates for available therapy. The median age was 68 years (range 40 to 91 years) with 61% of patients older than 65 years and 54% were male. Fifty percent (50%) of patients had primary MF, 31% had post-polycythemia vera MF and 18% had post-essential thrombocythemia MF. Twenty-one percent (21%) of patients had red blood cell transfusions within 8 weeks of enrollment in the study. The median hemoglobin count was 10.5 g/dL and the median platelet count was 251 X 109/L. Patients had a median palpable spleen length of 16 cm below the costal margin, with 81% having a spleen length 10 cm or greater below the costal margin. Patients had a median spleen volume as measured by magnetic resonance imaging (MRI) or computed tomography (CT) of 2595 cm3 (range 478 cm3 to 8881 cm3). (The upper limit of normal is approximately 300 cm3).
Patients were dosed with Jakafi or matching placebo. The primary efficacy endpoint was the proportion of patients achieving greater than or equal to a 35% reduction from baseline in spleen volume at Week 24 as measured by MRI or CT.
Secondary endpoints included duration of a 35% or greater reduction in spleen volume and proportion of patients with a 50% or greater reduction in Total Symptom Score from baseline to Week 24 as measured by the modified Myelofibrosis Symptom Assessment Form (MFSAF) v2.0 diary.
Study 2
Study 2 (NCT00934544) was an open-label, randomized study in 219 patients. Patients were randomized 2:1 to Jakafi versus best available therapy. Best available therapy was selected by the investigator on a patient-by-patient basis. In the best available therapy arm, the medications received by more than 10% of patients were hydroxyurea (47%) and glucocorticoids (16%). The median age was 66 years (range 35 to 85 years) with 52% of patients older than 65 years and 57% were male. Fifty-three percent (53%) of patients had primary MF, 31% had post-polycythemia vera MF and 16% had post-essential thrombocythemia MF. Twenty-one percent (21%) of patients had red blood cell transfusions within 8 weeks of enrollment in the study. The median hemoglobin count was 10.4 g/dL and the median platelet count was 236 X 109/L. Patients had a median palpable spleen length of 15 cm below the costal margin, with 70% having a spleen length 10 cm or greater below the costal margin. Patients had a median spleen volume as measured by MRI or CT of 2381 cm3 (range 451 cm3 to 7765 cm3).
The primary efficacy endpoint was the proportion of patients achieving 35% or greater reduction from baseline in spleen volume at Week 48 as measured by MRI or CT.
A secondary endpoint in Study 2 was the proportion of patients achieving a 35% or greater reduction of spleen volume as measured by MRI or CT from baseline to Week 24.
Study 1 and 2 Efficacy Results
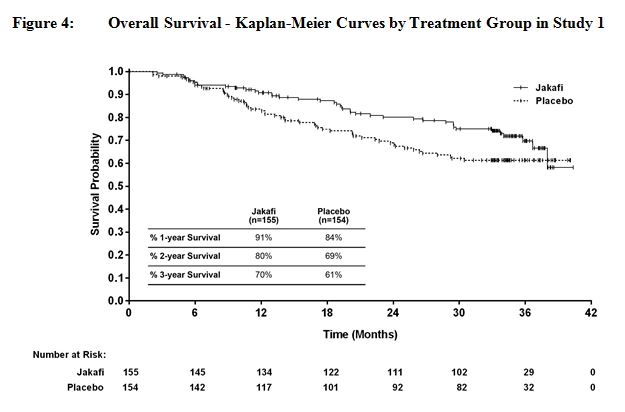
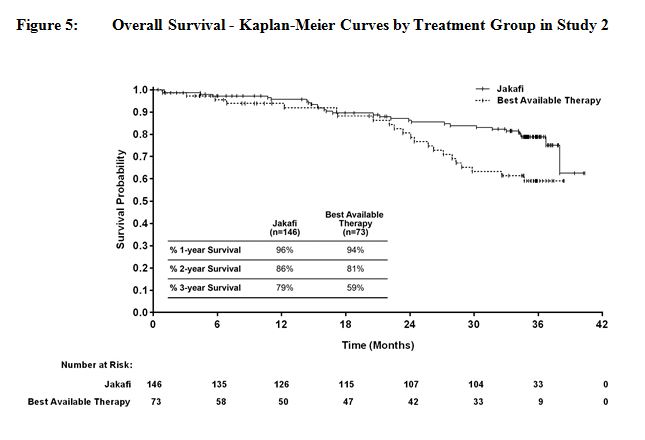
Efficacy analyses of the primary endpoint in Studies 1 and 2 are presented in Table 17 below. A significantly larger proportion of patients in the Jakafi group achieved a 35% or greater reduction in spleen volume from baseline in both studies compared to placebo in Study 1 and best available therapy in Study 2. A similar proportion of patients in the Jakafi group achieved a 50% or greater reduction in palpable spleen length.
Table 17: Percent of Patients with Myelofibrosis Achieving 35% or Greater Reduction from Baseline in Spleen Volume at Week 24 in Study 1 and at Week 48 in Study 2 (Intent to Treat) Study 1 Study 2 Jakafi
(N=155)Placebo
(N=154)Jakafi
(N=146)Best Available
Therapy
(N=73)Time Points Week 24 Week 48 Number (%) of Patients with
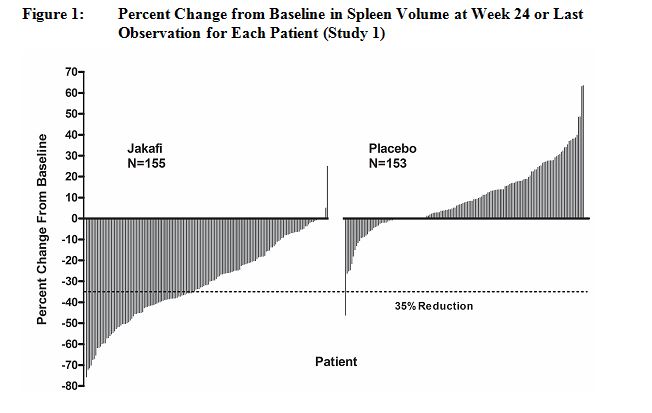
Spleen Volume Reduction by
35% or More65 (42) 1 (<1) 41 (29) 0 P-value < 0.0001 < 0.0001 Figure 1 shows the percent change from baseline in spleen volume for each patient at Week 24 (Jakafi N=139, placebo N=106) or the last evaluation prior to Week 24 for patients who did not complete 24 weeks of randomized treatment (Jakafi N=16, placebo N=47). One (1) patient (placebo) with a missing baseline spleen volume is not included.
In Study 1, MF symptoms were a secondary endpoint and were measured using the modified Myelofibrosis Symptom Assessment Form (MFSAF) v2.0 diary. The modified MFSAF is a daily diary capturing the core symptoms of MF (abdominal discomfort, pain under left ribs, night sweats, itching, bone/muscle pain and early satiety). Symptom scores ranged from 0 to 10 with 0 representing symptoms "absent" and 10 representing "worst imaginable" symptoms. These scores were added to create the daily total score, which has a maximum of 60.
Table 18 presents assessments of Total Symptom Score from baseline to Week 24 in Study 1 including the proportion of patients with at least a 50% reduction (ie, improvement in symptoms). At baseline, the mean Total Symptom Score was 18.0 in the Jakafi group and 16.5 in the placebo group. A higher proportion of patients in the Jakafi group had a 50% or greater reduction in Total Symptom Score than in the placebo group, with a median time to response of less than 4 weeks.
Table 18: Improvement in Total Symptom Score in Patients with Myelofibrosis Jakafi
(N=148)Placebo
(N=152)Number (%) of Patients with 50% or Greater Reduction
in Total Symptom Score by Week 2468 (46) 8 (5) P-value < 0.0001 Figure 2 shows the percent change from baseline in Total Symptom Score for each patient at Week 24 (Jakafi N=129, placebo N=103) or the last evaluation on randomized therapy prior to Week 24 for patients who did not complete 24 weeks of randomized treatment (Jakafi N=16, placebo N=42). Results are excluded for 5 patients with a baseline Total Symptom Score of zero, 8 patients with missing baseline and 6 patients with insufficient post-baseline data.
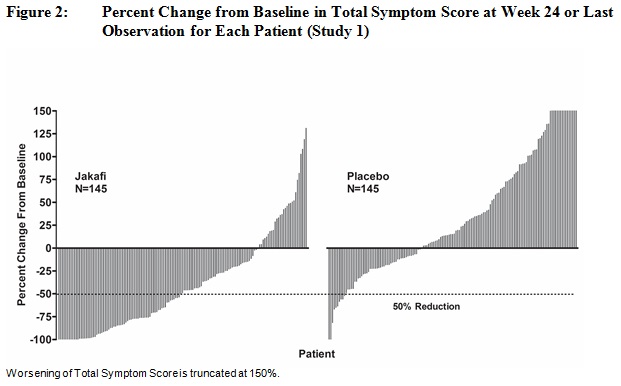
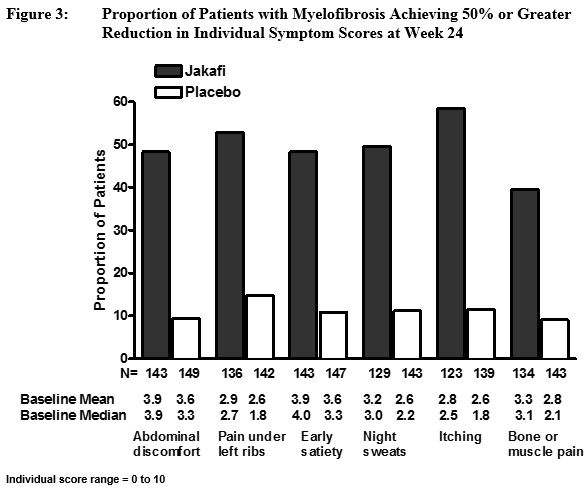
Figure 3 displays the proportion of patients with at least a 50% improvement in each of the individual symptoms that comprise the Total Symptom Score indicating that all 6 of the symptoms contributed to the higher Total Symptom Score response rate in the group treated with Jakafi.
An exploratory analysis of patients receiving Jakafi also showed improvement in fatigue-related symptoms (i.e., tiredness, exhaustion, mental tiredness, and lack of energy) and associated impacts on daily activities (i.e., activity limitations related to work, self-care, and exercise) as measured by the PROMIS® Fatigue 7-item short form total score at Week 24. Patients who achieved a reduction of 4.5 points or more from baseline to Week 24 in the PROMIS® Fatigue total score were considered to have achieved a fatigue response. Fatigue response was reported in 35% of patients in the Jakafi group versus 14% of the patients in the placebo group.
Overall survival was a secondary endpoint in both Study 1 and Study 2. Patients in the control groups were eligible for crossover in both studies, and the median times to crossover were 9 months in Study 1 and 17 months in Study 2.
Figure 4 and Figure 5 show Kaplan-Meier curves of overall survival at prospectively planned analyses after all patients remaining on study had completed 144 weeks on study.
14.2 Polycythemia Vera
Study 3 (NCT01243944) was a randomized, open-label, active-controlled Phase 3 study conducted in 222 patients with PV. Patients had been diagnosed with PV for at least 24 weeks, had an inadequate response to or were intolerant of hydroxyurea, required phlebotomy and exhibited splenomegaly. All patients were required to demonstrate hematocrit control between 40-45% prior to randomization. The age ranged from 33 to 90 years with 30% of patients over 65 years of age and 66% were male. Patients had a median spleen volume as measured by MRI or CT of 1272 cm3 (range 254 cm3 to 5147 cm3) and median palpable spleen length below the costal margin was 7 cm.
Patients were randomized to Jakafi or best available therapy. The starting dose of Jakafi was 10 mg twice daily. Doses were then individualized based upon tolerability and efficacy with a maximum dose of 25 mg twice daily. At Week 32, 98 patients were still on Jakafi with 8% receiving greater than 20 mg twice daily, 15% receiving 20 mg twice daily, 33% receiving 15 mg twice daily, 34% receiving 10 mg twice daily, and 10% receiving less than 10 mg twice daily. Best available therapy (BAT) was selected by the investigator on a patient-by-patient basis and included hydroxyurea (60%), interferon/pegylated interferon (12%), anagrelide (7%), pipobroman (2%), lenalidomide/thalidomide (5%), and observation (15%).
The primary endpoint was the proportion of subjects achieving a response at Week 32, with response defined as having achieved both hematocrit control (the absence of phlebotomy eligibility beginning at the Week 8 visit and continuing through Week 32) and spleen volume reduction (a greater than or equal to 35% reduction from baseline in spleen volume at Week 32). Phlebotomy eligibility was defined as a confirmed hematocrit greater than 45% that is at least 3 percentage points higher than the hematocrit obtained at baseline or a confirmed hematocrit greater than 48%, whichever was lower. Secondary endpoints included the proportion of all randomized subjects who achieved the primary endpoint and who maintained their response 48 weeks after randomization, and the proportion of subjects achieving complete hematological remission at Week 32 with complete hematological remission defined as achieving hematocrit control, platelet count less than or equal to 400 X 109/L, and white blood cell count less than or equal to 10 X 109/L.
Results of the primary and secondary endpoints are presented in Table 19. A significantly larger proportion of patients on the Jakafi arm achieved a response for the primary endpoint compared to best available therapy at Week 32 and maintained their response 48 weeks after randomization. A significantly larger proportion of patients on the Jakafi arm compared to best available therapy also achieved complete hematological remission at Week 32.
Table 19: Percent of Patients with Polycythemia Vera Achieving the Primary and Key Secondary Endpoints in Study 3 (Intent to Treat) Primary Response defined as having achieved both the absence of phlebotomy eligibility beginning at the Week 8 visit and continuing through Week 32 and a greater than or equal to 35% reduction from baseline in spleen volume at Week 32. Jakafi
(N=110)Best Available
Therapy
(N=112)Number (%) of Patients Achieving a Primary Response at Week 32 25 (23%) 1 (<1%) 95% CI of the response rate (%) (15%, 32%) (0%, 5%) P-value < 0.0001 Number (%) of Patients Achieving a Durable Primary Response at Week 48 22 (20%) 1 (<1%) 95% CI of the response rate (%) (13%, 29%) (0%, 5%) P-value < 0.0001 Number (%) of Patients Achieving Complete Hematological Remission at Week 32 26 (24%) 9 (8%) 95% CI of the response rate (%) (16%, 33%) (4%, 15%) P-value 0.0016 Additional analyses for Study 3 to assess durability of response were conducted at Week 80 only in the Jakafi arm. On this arm, 91 (83%) patients were still on treatment at the time of the Week 80 data cut-off. Of the 25 patients who achieved a primary response at Week 32, 19 (76% of the responders) maintained their response through Week 80, and of the 26 patients who achieved complete hematological remission at Week 32, 15 (58% of the responders) maintained their response through Week 80.
In an assessment of the individual components that make up the primary endpoint, there were 66 (60%) patients with hematocrit control on the Jakafi arm vs. 21 (19%) patients on best available therapy at Week 32; 51 (77% of hematocrit responders) patients on the Jakafi arm maintained hematocrit control through Week 80. There were 44 (40%) patients with spleen volume reduction from baseline greater than or equal to 35% on the Jakafi arm vs. 1 (<1%) patient on best available therapy at Week 32; 43 (98% of spleen volume reduction responders) patients on the Jakafi arm maintained spleen volume reduction through Week 80.
14.3 Acute Graft-Versus-Host Disease
Study 4 (NCT02953678) was an open-label, single-arm, multicenter study of Jakafi for treatment of patients with steroid-refractory acute GVHD Grades 2 to 4 (Mount Sinai Acute GVHD International Consortium (MAGIC) criteria) occurring after allogeneic hematopoietic stem cell transplantation. Jakafi was administered at 5 mg twice daily, and the dose could be increased to 10 mg twice daily after 3 days in the absence of toxicity.
There were 49 patients with acute GVHD refractory to steroids alone. These patients had a median age of 57 years (range, 18-72 years), 47% were male, 92% were Caucasian, and 14% were Hispanic. At baseline, acute GVHD was Grade 2 in 27%, Grade 3 in 55%, and Grade 4 in 18%; 84% had visceral GVHD; the median MAGIC biomarker score was 0.47 (range, 0.10‑0.92); and the median ST2 level was 334 mcg/L (range, 55-1286 mcg/L). The median duration of prior corticosteroid exposure at baseline was 15 days (range: 3 - 106 days).
The efficacy of Jakafi was based on Day-28 overall response rate (ORR) (complete response, very good partial response or partial response by Center for International Blood and Marrow Transplant Research (CIBMTR) criteria) and the duration of response. The ORR results are presented in Table 20; Day-28 ORR was 100% for Grade 2 GVHD, 40.7% for Grade 3 GVHD, and 44.4% for Grade 4 GVHD. The median duration of response, calculated from Day-28 response to progression, new salvage therapy for acute GVHD or death from any cause (with progression being defined as worsening by one stage in any organ without improvement in other organs in comparison to prior response assessment) was 16 days (95% CI 9, 83). Also for the Day-28 responders, the median time from Day-28 response to either death or need for new therapy for acute GVHD (additional salvage therapy or increase in steroids) was 173 days (95% CI 66, NE).
Table 20: Day-28 Overall Response Rate for Patients with Steroid-Refractory Acute GVHD in Study 4 Refractory to Steroids Alone
(n=49)Overall Response (%) (95% CI) 28 (57.1%) (42.2, 71.2) Complete Response 15 (30.6%) Very Good Partial Response 2 (4.1%) Partial Response 11 (22.4%) -
16. HOW SUPPLIED/STORAGE AND HANDLING
Jakafi (ruxolitinib) Tablets are available as follows:
Jakafi Trade Presentations Store at room temperature 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature]. NDC Number Strength Description Tablets per
Bottle50881-005-60 5 mg Round tablet with “INCY” on one side and “5” on the other 60 50881-010-60 10 mg Round tablet with “INCY” on one side and “10” on the other 60 50881-015-60 15 mg Oval tablet with “INCY” on one side and “15” on the other 60 50881-020-60 20 mg Capsule shaped tablet with “INCY” on one side and “20” on the other 60 50881-025-60 25 mg Oval tablet with “INCY” on one side and “25” on the othe 60 -
17. PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
Discuss the following with patients prior to and during treatment with Jakafi:
Thrombocytopenia, Anemia and Neutropenia
Inform patients that Jakafi is associated with thrombocytopenia, anemia and neutropenia, and of the need to monitor complete blood counts before and during treatment. Advise patients to observe for and report bleeding.
Infections
Inform patients of the signs and symptoms of infection and to report any such signs and symptoms promptly.
Inform patients regarding the early signs and symptoms of herpes zoster and of progressive multifocal leukoencephalopathy, and advise patients to seek advice of a clinician if such symptoms are observed.
Symptom Exacerbation Following Interruption or Discontinuation of Treatment with Jakafi
Inform patients that after discontinuation of treatment, signs and symptoms from myeloproliferative neoplasms are expected to return. Instruct patients not to interrupt or discontinue Jakafi therapy without consulting their physician.
Non-Melanoma Skin Cancer
Inform patients that Jakafi may increase their risk of certain non-melanoma skin cancers. Advise patients to inform their healthcare provider if they have ever had any type of skin cancer or if they observe any new or changing skin lesions.
Lipid Elevations
Inform patients that Jakafi may increase blood cholesterol, and of the need to monitor blood cholesterol levels.
Drug-drug Interactions
Advise patients to inform their healthcare providers of all medications they are taking, including over-the-counter medications, herbal products and dietary supplements.
Dialysis
Inform patients on dialysis that their dose should not be taken before dialysis but only following dialysis.
Lactation
Inform women not to breastfeed during treatment with Jakafi and for two weeks after the final dose
Compliance
Advise patients to continue taking Jakafi every day for as long as their physician tells them and that this is a long-term treatment. Patients should not change dose or stop taking Jakafi without first consulting their physician. Patients should be aware that after discontinuation of treatment, signs and symptoms from myeloproliferative neoplasms are expected to return.
Manufactured for:
Incyte Corporation
1801 Augustine Cut-off
Wilmington, DE 19803Jakafi is a registered trademark of Incyte. All rights reserved.
U.S. Patent Nos. 7598257; 8415362; 8722693; 8822481; 8829013; 9079912; 9814722; 10016429
© 2011-2020 Incyte Corporation. All rights reserved. -
PATIENT PACKAGE INSERT
Patient Information
JAKAFI® (JAK-ah-fye)
(ruxolitinib)
tablets
What is Jakafi?
Jakafi is a prescription medicine used to treat:
- adults with certain types of myelofibrosis (MF).
- adults with polycythemia vera (PV) who have already taken a medicine called hydroxyurea and it did not work well enough or they could not tolerate it
- adults and children 12 years of age and older with acute graft versus host disease (GVHD) who have taken corticosteroids and they did not work well enough.
It is not known if Jakafi is safe or effective in children for treatment of myelofibrosis or polycythemia vera.
Before taking Jakafi, tell your healthcare provider about of your medical conditions, including if you:
- have an infection
- have or had tuberculosis (TB), or have been in close contact with someone who has TB
- have or had hepatitis B
- have or have had liver problems
- have or have had kidney problems or are on dialysis. If you are on dialysis, Jakafi should be taken after your dialysis
- have high level of fat in your blood (high blood cholesterol or triglycerides)
- have had skin cancer in the past
- are pregnant or plan to become pregnant. It is not known if Jakafi will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Jakafi passes into your breast milk. Do not breastfeed during treatment with Jakafi and for 2 weeks after the final dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Taking Jakafi with certain other medicines may affect how Jakafi works. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Jakafi? - Take Jakafi exactly as your healthcare provider tells you.
- Do not change your dose or stop taking Jakafi without first talking to your healthcare provider.
- You can take Jakafi with or without food.
- Jakafi may also be given through certain nasogastric tubes.
- Tell your healthcare provider if you cannot take Jakafi by mouth. Your healthcare provider will decide if you can take Jakafi through a nasogastric tube.
- Ask your healthcare provider to give you specific instruction on how to properly take Jakafi through a nasogastric tube.
- If you miss a dose of Jakafi, take your next dose at your regular time. Do not take 2 doses at the same time.
- If you take too much Jakafi call your healthcare provider or go to the nearest hospital emergency room right away.
- You will have regular blood tests during your treatment with Jakafi. Your healthcare provider may change your dose of Jakafi or stop your treatment based on the results of your blood tests.
What are the possible side effects of Jakafi?
Jakafi can cause serious side effects including:
Low blood cell counts. Jakafi may cause low platelet counts (thrombocytopenia), low red blood cell counts (anemia), and low white blood cell counts (neutropenia). If you develop bleeding, stop Jakafi and call your healthcare provider. Your healthcare provider will do a blood test to check your blood cell counts before you start Jakafi and regularly during your treatment with Jakafi. Tell your healthcare provider right away if you develop or have worsening of any of these symptoms:- unusual bleeding
- bruising
- tiredness
- shortness of breath
- fever
Infection. You may be at risk for developing a serious infection during treatment with Jakafi. Tell your healthcare provider if you develop any of the following symptoms of infection:
- chills
- aches
- fever
- nausea
- vomiting
- weakness
- painful skin rash or blisters
Skin cancers. Some people who take Jakafi have developed certain types of non-melanoma skin cancers. Tell your healthcare provider if you develop any new or changing skin lesions during treatment with Jakafi.
Cholesterol increases. You may have changes in your blood cholesterol levels. Your healthcare provider will do blood tests to check your cholesterol levels during treatment with Jakafi.
The most common side effects of Jakafi in adults with certain types of MF and PV include:- low platelet counts (thrombocytopenia)
- low red blood cell counts (anemia)
- bruising
- dizziness
- headache
- diarrhea
The most common side effects of Jakafi in people with acute graft versus host disease (GVHD) include:
- low red blood cell counts (anemia)
- low platelet counts (thrombocytopenia)
- low white blood cell counts (neutropenia)
- infections
- fluid retention
These are not all the possible side effects of Jakafi.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Incyte Corporation at 1-855-463-3463.How should I store Jakafi?
- Store Jakafi at room temperature 68°F to 77°F (20°C to 25°C).
Keep Jakafi and all medicines out of the reach of children.
General information about the safe and effective use of Jakafi.
Medicines are sometimes prescribed for purposes other than those listed in Patient Information. Do not use Jakafi for a condition for which it is not prescribed. Do not give Jakafi to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information that is written for healthcare professionals.
What are the ingredients in Jakafi?
Active ingredient: ruxolitinib phosphate
Inactive ingredients: microcrystalline cellulose, lactose monohydrate, magnesium stearate, colloidal silicon dioxide, sodium starch glycolate, povidone and hydroxypropyl cellulose
Manufactured for: Incyte Corporation, 1801 Augustine Cut-off, Wilmington, DE 19803
Jakafi is a registered trademark of Incyte. All rights reserved.
U.S. Patent Nos. 7598257; 8415362; 8722693; 8822481; 8829013; 9079912; 9814722; 10016429
© 2011-2020 Incyte Corporation. All rights reserved.
For more information call 1-855-463-3463 or go to www.jakafi.com.This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: January 2020
-
5 mg Tablet Bottle Label
Rx only
NDC: 50881-005-60
Jakafi® (Ruxolitinib) Tablets
5 mg
60 tablets
Each tablet contains ruxolitinib phosphate equivalent to 5 mg ruxolitinib free base.

-
10 mg Tablet Bottle Label
Rx only
NDC: 50881-010-60
Jakafi® (Ruxolitinib) Tablets
10 mg
60 tablets
Each tablet contains ruxolitinib phosphate equivalent to 10 mg ruxolitinib free base.

-
15 mg Tablet Bottle Label
Rx only
NDC: 50881-015-60
Jakafi® (Ruxolitinib) Tablets
15 mg
60 tablets
Each tablet contains ruxolitinib phosphate equivalent to 15 mg ruxolitinib free base.

-
20 mg Tablet Bottle Label
Rx only
NDC: 50881-020-60
Jakafi® (Ruxolitinib) Tablets
20 mg
60 tablets
Each tablet contains ruxolitinib phosphate equivalent to 20 mg ruxolitinib free base.

-
25 mg Tablet Bottle Label
Rx only
NDC: 50881-025-60
Jakafi® (Ruxolitinib) Tablets
25 mg
60 tablets
Each tablet contains ruxolitinib phosphate equivalent to 25 mg ruxolitinib free base.

-
INGREDIENTS AND APPEARANCE
JAKAFI
ruxolitinib tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50881-005 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RUXOLITINIB (UNII: 82S8X8XX8H) (RUXOLITINIB - UNII:82S8X8XX8H) RUXOLITINIB 5.0 mg Product Characteristics Color WHITE (White to off-white) Score no score Shape ROUND Size 7mm Flavor Imprint Code INCY;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50881-005-60 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/16/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202192 11/16/2011 JAKAFI
ruxolitinib tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50881-010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RUXOLITINIB (UNII: 82S8X8XX8H) (RUXOLITINIB - UNII:82S8X8XX8H) RUXOLITINIB 10.0 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROXYPROPYL CELLULOSE (70000 WAMW) (UNII: 66O7AQV0RT) POVIDONE (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (White to off-white) Score no score Shape ROUND Size 9mm Flavor Imprint Code INCY;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50881-010-01 28 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/16/2011 2 NDC: 50881-010-60 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/16/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202192 11/16/2011 JAKAFI
ruxolitinib tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50881-015 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RUXOLITINIB (UNII: 82S8X8XX8H) (RUXOLITINIB - UNII:82S8X8XX8H) RUXOLITINIB 15.0 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROXYPROPYL CELLULOSE (70000 WAMW) (UNII: 66O7AQV0RT) POVIDONE (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (White to off-white) Score no score Shape OVAL Size 15mm Flavor Imprint Code INCY;15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50881-015-60 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/16/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202192 11/16/2011 JAKAFI
ruxolitinib tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50881-020 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RUXOLITINIB (UNII: 82S8X8XX8H) (RUXOLITINIB - UNII:82S8X8XX8H) RUXOLITINIB 20.0 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROXYPROPYL CELLULOSE (70000 WAMW) (UNII: 66O7AQV0RT) POVIDONE (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (White to off-white) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code INCY;20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50881-020-60 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/16/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202192 11/16/2011 JAKAFI
ruxolitinib tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50881-025 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RUXOLITINIB (UNII: 82S8X8XX8H) (RUXOLITINIB - UNII:82S8X8XX8H) RUXOLITINIB 25.0 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROXYPROPYL CELLULOSE (70000 WAMW) (UNII: 66O7AQV0RT) POVIDONE (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (White to off-white) Score no score Shape OVAL Size 18mm Flavor Imprint Code INCY;25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50881-025-60 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/16/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202192 11/16/2011 Labeler - Incyte Corporation (556967347)
Trademark Results [JAKAFI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 JAKAFI 85569591 4267302 Live/Registered |
INCYTE HOLDINGS CORPORATION 2012-03-14 |
 JAKAFI 85280226 4126784 Live/Registered |
INCYTE HOLDINGS CORPORATION 2011-03-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.