Tazarotene by Solaris Pharma Corporation TAZAROTENE gel
Tazarotene by
Drug Labeling and Warnings
Tazarotene by is a Prescription medication manufactured, distributed, or labeled by Solaris Pharma Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TAZAROTENE GEL safely and effectively. See full prescribing information for TAZAROTENE GEL.
TAZAROTENE gel, 0.05% and 0.1%, for topical use
Initial U.S. Approval: 1997
INDICATIONS AND USAGE
- Tazarotene gel, 0.05% and 0.1% is a retinoid indicated for the topical treatment of plaque psoriasis of up to 20% body surface area involvement. (1.1)
- Tazarotene gel, 0.1% is indicated for the topical treatment of mild to moderate facial acne vulgaris. (1.2)
Limitations of Use
- The safety of tazarotene gel use on more than 20% body surface area has not been established. (1.3)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Gel, 0.05% and 0.1% (3)
WARNINGS AND PRECAUTIONS
- Embryofetal Toxicity: Tazarotene gel contains tazarotene, which is a teratogen. Tazarotene gel is contraindicated in pregnancy. Females of child-bearing potential should have a negative pregnancy test within 2 weeks prior to initiating treatment and use an effective method of contraception during treatment. (5.1)
- Local Irritation: Excessive pruritus, burning, skin redness or peeling can occur. If these reactions occur, discontinue until the integrity of the skin has been restored, or consider reducing dosing frequency or in the case of psoriasis, consider switching to the lower concentration. Tazarotene gel should not be used on eczematous skin, as it may cause severe irritation. (5.2)
- Photosensitivity and Risk for Sunburn: Avoid exposure to sunlight, sunlamps, and weather extremes. Wear sunscreen daily. Tazarotene gel should be administered with caution if the patient is also taking drugs known to be photosensitizers. (5.3)
ADVERSE REACTIONS
- Plaque Psoriasis: Most common adverse reactions occurring in 10 to 30% of patients are pruritus, burning/stinging, erythema, worsening of psoriasis, irritation, and skin pain. (6.1)
- Acne Vulgaris: Most common adverse reactions occurring in 10 to 30% of patients are desquamation, burning/stinging, dry skin, erythema and pruritus. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Solaris Pharma Corporation at 1-833-919-0527 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Plaque Psoriasis
1.2 Acne Vulgaris
1.3 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Psoriasis
2.2 Acne
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Embryofetal Toxicity
5.2 Local Irritation and Hypersensitivity Reactions
5.3 Photosensitivity and Risk for Sunburn
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.2 FDA-Approved Patient Labeling
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Plaque Psoriasis
Tazarotene gel, 0.05% and 0.1% are indicated for the topical treatment of patients with plaque psoriasis of up to 20% body surface area involvement.
1.2 Acne Vulgaris
Tazarotene gel, 0.1% is also indicated for the topical treatment of patients with facial acne vulgaris of mild to moderate severity.
The efficacy of tazarotene gel in the treatment of acne previously treated with other retinoids or resistant to oral antibiotics has not been established.
-
2 DOSAGE AND ADMINISTRATION
Tazarotene gel is for topical use only. Tazarotene gel is not for ophthalmic, oral, or intravaginal use. Avoid accidental transfer of tazarotene gel into eyes, mouth, or other mucous membranes. If contact with mucous membranes occurs, rinse thoroughly with water [see Warnings and Precautions (5.2)].
Wash hands thoroughly after application.
2.1 Psoriasis
It is recommended that treatment starts with tazarotene gel, 0.05%, with strength increased to 0.1% if tolerated and medically indicated. Apply a thin film (2 mg/cm2) of tazarotene gel once per day, in the evening, to cover only the psoriatic lesions on no more than 20% of body surface area. If a bath or shower is taken prior to application, the skin should be dry before applying the gel. If emollients are used, they should be applied at least an hour before application of tazarotene gel. Because unaffected skin may be more susceptible to irritation, application of tazarotene to these areas should be carefully avoided. Tazarotene gel was investigated for up to 12 months during clinical trials for psoriasis.
2.2 Acne
Cleanse the face gently. After the skin is dry, apply a thin layer (2 mg/cm2) of tazarotene gel 0.1% once per day, in the evening, to the skin where acne lesions appear. Use enough to cover the entire affected area. Tazarotene gel was investigated for up to 12 weeks during clinical trials for acne. Use effective sunscreens and wear protective clothing while using tazarotene gel [see Warnings and Precautions (5.3)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Tazarotene gel is contraindicated in:
- Pregnancy. Retinoids may cause fetal harm when administered to a pregnant female [see Warnings and Precautions (5.1), Use in Specific Populations (8.1, 8.3)].
- Individuals who have known hypersensitivity to any of its components [see Warnings and Precautions (5.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Embryofetal Toxicity
Based on data from animal reproduction studies, retinoid pharmacology and the potential for systemic absorption, tazarotene gel may cause fetal harm when administered to a pregnant female and is contraindicated during pregnancy. Tazarotene elicits malformations and developmental effects associated with retinoids after topical and oral administration to pregnant rats and rabbits during organogenesis.
Systemic exposure to tazarotenic acid is dependent upon the extent of the body surface area treated. In patients treated topically over sufficient body surface area, exposure could be in the same order of magnitude as in orally treated animals.
Although there may be less systemic exposure in the treatment of acne of the face alone due to less surface area for application, tazarotene is a teratogenic substance and causes fetal malformations in animals, and it is not known what level of exposure is required for teratogenicity in humans [see Clinical Pharmacology (12.3)].
There were thirteen reported pregnancies in subjects who participated in the clinical trials for topical tazarotene. Nine of the subjects had been treated with topical tazarotene, and the other four had been treated with vehicle. One of the subjects who was treated with tazarotene cream elected to terminate the pregnancy for non-medical reasons unrelated to treatment. The other eight pregnant women who were inadvertently exposed to topical tazarotene during the clinical trials subsequently delivered apparently healthy babies. As the exact timing and extent of exposure in relation to the gestation times are not certain, the significance of these findings is unknown.
Females of Child-bearing Potential
Females of child-bearing potential should be warned of the potential risk and use adequate birth-control measures when tazarotene gel is used. The possibility that a female of child-bearing potential is pregnant at the time of institution of therapy should be considered.A negative result for pregnancy test should be obtained within 2 weeks prior to tazarotene gel therapy. Tazarotene gel therapy should begin during a normal menstrual period [see Use in Specific Populations (8.1)].
5.2 Local Irritation and Hypersensitivity Reactions
Application of tazarotene gel may cause excessive irritation in the skin of certain sensitive individuals. Local reactions (including blistering and skin desquamation, pruritus, burning, erythema) and hypersensitivity adverse reactions (including urticaria) have been observed with topical tazarotene.
If these adverse reactions occur, consider discontinuing the medication or reducing the dosing frequency, as appropriate, until the integrity of the skin is restored. Alternatively, patients with psoriasis who are being treated with the 0.1% concentration can be switched to the lower concentration. Frequency of application should be closely monitored by careful observation of the clinical therapeutic response and skin tolerance. Therapy can be resumed, or the drug concentration or frequency of application can be increased as the patient becomes able to tolerate treatment.
Concomitant topical medications and cosmetics that have a strong drying effect should be avoided. It is also advisable to "rest" a patient's skin until the effects of such preparations subside before treatment with tazarotene gel is initiated.
Tazarotene gel, should not be used on eczematous skin, as it may cause severe irritation.
Weather extremes, such as wind or cold, may be more irritating to patients using tazarotene gel.
5.3 Photosensitivity and Risk for Sunburn
Because of heightened burning susceptibility, exposure to sunlight (including sunlamps) should be avoided unless deemed medically necessary, and in such cases, exposure should be minimized during the use of tazarotene gel. Patients must be warned to use sunscreens and protective clothing when using tazarotene gel. Patients with sunburn should be advised not to use tazarotene gel until fully recovered. Patients who may have considerable sun exposure due to their occupation and those patients with inherent sensitivity to sunlight should exercise particular caution when using tazarotene gel.
Tazarotene gel should be administered with caution if the patient is also taking drugs known to be photosensitizers (e.g., thiazides, tetracyclines, fluoroquinolones, phenothiazines, sulfonamides) because of the increased possibility of augmented photosensitivity.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in more detail in other sections of the labeling:
- Embryofetal toxicity [see Warnings and Precautions (5.1)]
- Photosensitivity and Risk of Sunburn [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Psoriasis
A total of 439 subjects 14 to 87 years of age were treated with tazarotene gel, 0.05% and 0.1% in two controlled clinical trials. The most frequent adverse events reported with tazarotene gel, 0.05% and 0.1% occurring in 10 to 30% of subjects, in descending order, included pruritus, burning/stinging, erythema, worsening of psoriasis, irritation, and skin pain. Reactions occurring in 1 to 10% of subjects included rash, desquamation, irritant contact dermatitis, skin inflammation, fissuring, bleeding, and dry skin. Increases in "psoriasis worsening" and "sun-induced erythema" were noted in some subjects over the 4th to 12th months of treatment as compared to the first three months of a 1 year study. In general, the incidence of adverse events with tazarotene gel 0.05% was 2 to 5% lower than that seen with tazarotene gel 0.1%.Acne
A total of 596 subjects 12 to 44 years of age were treated with tazarotene gel, 0.05% and 0.1% in two controlled clinical trials. The most frequent adverse events reported during clinical trials with tazarotene gel, 0.1% in the treatment of acne occurring in 10 to 30% of subjects, in descending order, included desquamation, burning/stinging, dry skin, erythema and pruritus. Reactions occurring in 1 to 10% of subjects included irritation, skin pain, fissuring, localized edema and skin discoloration.6.2 Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following adverse reactions have been identified during postapproval use of tazarotene.
Skin and subcutaneous tissue disorders: blister, dermatitis, urticaria, skin exfoliation, skin discoloration (including skin hyperpigmentation or skin hypopigmentation), swelling at or near application sites, and pain.
-
7 DRUG INTERACTIONS
No formal drug-drug interaction studies were conducted with tazarotene gel.
In a trial of 27 healthy female subjects between the ages of 20-55 years receiving a combination oral contraceptive tablet containing 1 mg norethindrone and 35 mcg ethinyl estradiol, concomitant use of tazarotene administered as 1.1 mg orally (mean ± SD Cmax and AUC0-24 of tazarotenic acid were 28.9 ± 9.4 ng/mL and 120.6 ± 28.5 ng·hr/mL, respectively) did not affect the pharmacokinetics of norethindrone and ethinyl estradiol over a complete cycle.
The impact of tazarotene on the pharmacokinetics of progestin only oral contraceptives (i.e., minipills) has not been evaluated.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on data from animal reproduction studies, retinoid pharmacology, and the potential for systemic absorption, tazarotene gel may cause fetal harm when administered to a pregnant female and is contraindicated during pregnancy. Safety in pregnant females has not been established. The potential risk to the fetus outweighs the potential benefit to the mother from tazarotene gel during pregnancy; therefore, tazarotene gel should be discontinued as soon as pregnancy is recognized [see Contraindications (4), Warnings and Precautions (5.1), Clinical Pharmacology (12.3)]. Limited case reports of pregnancy in females enrolled in clinical trials for tazarotene gel have not established a clear association with tazarotene and major birth defects or miscarriage risk. Because the exact timing and extent of exposure in relation to the gestational age are not certain, the significance of these findings is unknown.In animal reproduction studies with pregnant rats, tazarotene dosed topically during organogenesis at 0.5 times the maximum systemic exposure in subjects treated with the maximum recommended human dose (MRHD) of tazarotene gel, 0.1% resulted in reduced fetal body weights and reduced skeletal ossification. In animal reproduction studies with pregnant rabbits dosed topically with tazarotene gel at 7 times the maximum systemic exposure in subjects treated with the MRHD of tazarotene gel, 0.1%, there were single incidences of known retinoid malformations, including spina bifida, hydrocephaly, and heart anomalies.
In animal reproduction studies with pregnant rats and rabbits, tazarotene dosed orally during organogenesis at 0.5 and 13 times, respectively, the maximum systemic exposure in subjects treated with the MRHD of tazarotene gel, 0.1% resulted in malformations, fetal toxicity, developmental delays, and/or behavioral delays. In pregnant rats, tazarotene dosed orally prior to mating through early gestation resulted in decreased litter size, decreased numbers of live fetuses, decreased fetal body weights, and increased malformations at doses approximately 2 times higher than the maximum systemic exposure in subjects treated with the MRHD of tazarotene gel, 0.1% [see Data].
The background risk of major birth defects and miscarriage for the indicated population is unknown. Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In rats, a tazarotene gel, 0.05% formulation dosed topically during gestation days 6 through 17 at 0.25 mg/kg/day, which represented 0.5 times the maximum systemic exposure in subjects treated with the MRHD of tazarotene gel, 0.1% (i.e., 2 mg/cm2 over a 20% body surface area), resulted in reduced fetal body weights and reduced skeletal ossification. Rabbits dosed topically with 0.25 mg/kg/day tazarotene gel, which represented 7 times the maximum systemic exposure in subjects treated with the MRHD of tazarotene gel, 0.1%, during gestation days 6 through 18 were noted with single incidences of known retinoid malformations, including spina bifida, hydrocephaly, and heart anomalies.When tazarotene was given orally to animals, developmental delays were seen in rats, and malformations and post-implantation loss were observed in rats and rabbits at doses producing 0.5 and 13 times, respectively, the maximum systemic exposure in subjects treated with the MRHD of tazarotene gel, 0.1%.
In female rats orally administered 2 mg/kg/day of tazarotene from 15 days before mating through gestation day 7, which represented 2 times the maximum systemic exposure in subjects treated with the MRHD of tazarotene gel, 0.1%, classic developmental effects of retinoids were observed including decreased number of implantation sites, decreased litter size, decreased numbers of live fetuses, and decreased fetal body weights. A low incidence of retinoid-related malformations was observed at that dose.
In a pre- and postnatal development toxicity study, topical administration of tazarotene gel (0.125 mg/kg/day) to pregnant female rats from gestation day 16 through lactation day 20 reduced pup survival, but did not affect the reproductive capacity of the offspring. Based on data from another study, the maximum systemic exposure in the rat would be 0.3 times the maximum systemic exposure in subjects treated with the MRHD of tazarotene gel, 0.1%.
8.2 Lactation
Risk Summary
There is no information regarding the presence of tazarotene in human milk, the effects on the breastfed infant, or the effects on milk production. After single topical doses of 14C-tazarotene gel to the skin of lactating rats, radioactivity was detected in rat milk. The lack of clinical data during lactation precludes a clear determination of the risk of tazarotene gel to an infant during lactation; therefore, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for tazarotene gel and any potential adverse effects on the breastfed child from tazarotene gel or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential within 2 weeks prior to initiating tazarotene gel therapy which should begin during a menstrual period.Contraception
Females
Based on animal studies, tazarotene gel may cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with tazarotene gel.8.4 Pediatric Use
The safety and efficacy of tazarotene gel have not been established in pediatric patients with psoriasis or acne under the age of 12 years.
8.5 Geriatric Use
Of the total number of subjects in clinical trials of tazarotene gel for plaque psoriasis, 163 were over the age of 65. Subjects over 65 years of age experienced more adverse events and lower treatment success rates after 12 weeks of use of tazarotene gel compared with those 65 years of age and younger. Currently there is no other clinical experience on the differences in responses between the elderly and younger subjects, but greater sensitivity of some older individuals cannot be ruled out. Tazarotene gel for the treatment of acne has not been clinically evaluated in persons over the age of 65.
-
10 OVERDOSAGE
Excessive topical use of tazarotene gel, 0.05% and 0.1% may lead to marked redness, peeling, or discomfort [see Warnings and Precautions (5.2)].
Tazarotene gel, 0.05% and 0.1% are not for oral use. Oral ingestion of the drug may lead to the same adverse effects as those associated with excessive oral intake of Vitamin A (hypervitaminosis A) or other retinoids. If oral ingestion occurs, the patient should be monitored, and appropriate supportive measures should be administered as necessary.
-
11 DESCRIPTION
Tazarotene gel, 0.05% and 0.1% is for topical use and contains the active ingredient, tazarotene. Each gram of tazarotene gel, 0.05% and 0.1% contains 0.5 and 1 mg of tazarotene, respectively in a translucent, aqueous gel.
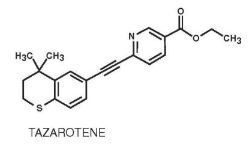
Tazarotene is a member of the acetylenic class of retinoids. Chemically, tazarotene is ethyl 6-[(4,4-dimethylthiochroman-6-yl)ethynyl]nicotinate. The compound has an empirical formula of C21H21NO2S and molecular weight of 351.46. The structural formula is shown below:
Tazarotene gel, 0.05% and 0.1% contains the following inactive ingredients:
benzyl alcohol 1%; ascorbic acid; butylated hydroxyanisole; butylated hydroxytoluene; carbomer homopolymer type B; edetate disodium; hexylene glycol; poloxamer 407; polyethylene glycol 400; polysorbate 40; purified water; and tromethamine. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tazarotene is a retinoid prodrug which is converted to its active form, the carboxylic acid of tazarotene, by deesterification. Tazarotenic acid binds to all three members of the retinoic acid receptor (RAR) family: RARα, RARβ, and RARγ, but shows relative selectivity for RARβ, and RARγ and may modify gene expression. The clinical significance of these findings for the treatment of plaque psoriasis and facial acne vulgaris is unknown.
12.2 Pharmacodynamics
The pharmacodynamics of tazarotene gel in the treatment of plaque psoriasis and facial acne vulgaris are unknown.
12.3 Pharmacokinetics
Following topical application, tazarotene undergoes esterase hydrolysis to form its active metabolite, tazarotenic acid. Little parent compound could be detected in the plasma. Tazarotenic acid was highly bound to plasma proteins (greater than 99%).
Tazarotene and tazarotenic acid were metabolized to sulfoxides, sulfones and other polar metabolites which were eliminated through urinary and fecal pathways. The half-life of tazarotenic acid was approximately 18 hours, following topical application of tazarotene to normal, acne or psoriatic skin.
The human in vivo studies described below were conducted with tazarotene gel applied topically at approximately 2 mg/cm2 and left on the skin for 10 to 12 hours. Both the peak plasma concentration (Cmax) and area under the plasma concentration time curve (AUC) refer to the active metabolite only.
Two single, topical dose studies were conducted using 14C-tazarotene gel. Systemic absorption, as determined from radioactivity in the excreta, was less than 1% of the applied dose (without occlusion) in six subjects with psoriasis and approximately 5% of the applied dose (under occlusion) in six healthy subjects. One non-radiolabeled single-dose study comparing the 0.05% gel to the 0.1% gel in healthy subjects indicated that the Cmax and AUC were 40% higher for the 0.1% gel.
After 7 days of topical dosing with measured doses of tazarotene 0.1% gel on 20% of the total body surface without occlusion in 24 healthy subjects, the Cmax for tazarotenic acid was 0.72 ± 0.58 ng/mL (mean ± SD) occurring 9 hours after the last dose, and the AUC0-24hr for tazarotenic acid was 10.1 ± 7.2 ng·hr/mL. Systemic absorption was 0.91 ± 0.67% of the applied dose.
In a 14-day study in five subjects with psoriasis, measured doses of tazarotene 0.1% gel were applied daily by nursing staff to involved skin without occlusion (8 to 18% of total body surface area; mean ± SD: 13 ± 5%). The Cmax for tazarotenic acid was 12.0 ± 7.6 ng/mL occurring 6 hours after the final dose, and the AUC0-24hr for tazarotenic acid was 105 ± 55 ng·hr/mL. Systemic absorption was 14.8 ± 7.6% of the applied dose. Extrapolation of these results to represent dosing on 20% of total body surface yielded estimates for tazarotenic acid with Cmax of 18.9 ± 10.6 ng/mL and AUC0-24hr of 172 ± 88 ng·hr/mL.
An in vitro percutaneous absorption study, using radiolabeled drug and freshly excised human skin or human cadaver skin, indicated that approximately 4 to 5% of the applied dose was in the stratum corneum (tazarotene: tazarotenic acid = 5:1) and 2 to 4% was in the viable epidermis-dermis layer (tazarotene: tazarotenic acid = 2:1) 24 hours after topical application of the gel.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A long-term study of tazarotene following oral administration of 0.025, 0.050, and 0.125 mg/kg/day to rats showed no indications of increased carcinogenic risks. Based on pharmacokinetic data from a shorter-term study in rats, the highest dose of 0.125 mg/kg/day was anticipated to give systemic exposure in the rat 0.3 times that seen in subjects treated with the MRHD of tazarotene gel, 0.1%.
A long-term study with topical administration of up to 0.1% tazarotene in a gel formulation in mice terminated at 88 weeks showed that dose levels of 0.05, 0.125, 0.25, and 1 mg/kg/day (reduced to 0.5 mg/kg/day for males after 41 weeks due to severe dermal irritation) revealed no apparent carcinogenic effects when compared to vehicle control animals. Systemic exposure at the highest dose was 2 times that seen in subjects treated with the MRHD of tazarotene gel, 0.1%.
Tazarotene was non-mutagenic in the Ames assay and did not produce structural chromosomal aberrations in a human lymphocyte assay. Tazarotene was non-mutagenic in the CHO/HGPRT mammalian cell forward gene mutation assay and was non-clastogenic in the in vivo mouse micronucleus test.
No impairment of fertility occurred in rats when male animals were treated for 70 days prior to mating and female animals were treated for 14 days prior to mating and continuing through gestation and lactation with topical doses of tazarotene gel up to 0.125 mg/kg/day. Based on data from another study, the systemic drug exposure in the rat at the highest dose was 0.3 times that observed in subjects treated with the MRHD of tazarotene gel, 0.1%.
No impairment of mating performance or fertility was observed in male rats treated for 70 days prior to mating with oral doses of up to 1 mg/kg/day tazarotene, which produced systemic exposure that was approximately equivalent to that observed in subjects treated with the MRHD of tazarotene gel, 0.1%.
No impairment of mating performance or fertility was observed in female rats treated for 15 days prior to mating and continuing through gestation day 7 with oral doses of tazarotene up to 2 mg/kg/day. However, there was a significant decrease in the number of estrous stages and an increase in developmental effects at that dose, which produced systemic exposure 2 times that observed in subjects treated with the MRHD of tazarotene gel, 0.1% [see Use in Specific Populations (8.1)].
-
14 CLINICAL STUDIES
Psoriasis: In two large vehicle-controlled clinical trials, tazarotene gel, 0.05% and 0.1% applied once daily for 12 weeks was significantly more effective than vehicle in reducing the severity of the clinical signs of plaque psoriasis covering up to 20% of body surface area. In one of the studies, subjects were followed up for an additional 12 weeks following cessation of therapy with tazarotene gel. Mean baseline scores and changes from baseline (reductions) after treatment in these two trials are shown in Table 1.
Table 1. Plaque Elevation, Scaling, and Erythema in Two Controlled Clinical Trials for Psoriasis Plaque elevation, scaling, and erythema scored on a 0-4 scale with 0=none, 1=mild, 2=moderate, 3=severe and 4=very severe.
B*=Mean Baseline Severity: C-12*=Mean Change from Baseline at end of 12 weeks of therapy:
C-24*=Mean Change from Baseline at week 24 (12 weeks after the end of therapy).
Tazarotene Gel, 0.05%
Tazarotene Gel, 0.1%
Vehicle Gel
Trunk/Arm/Leg
Lesions
Knee/Elbow
Lesions
Trunk/Arm/Leg
Lesions
Knee/Elbow
Lesions
Trunk/Arm/Leg
Lesions
Knee/Elbow
Lesions
N=108
N=111
N=108
N=111
N=108
N=112
N=108
N=112
N=108
N=113
N=108
N=113
Plaque
Elevation
B*
C-12*
C-24*
2.5
-1.4
-1.2
2.6
-1.3
2.6
-1.3
-1.1
2.6
-1.1
2.5
-1.4
-1.1
2.6
-1.4
2.6
-1.5
-1.0
2.6
-1.3
2.4
-0.8
-0.9
2.6
-0.7
2.6
-0.7
-0.7
2.6
-0.6
Scaling
B*
C-12*
C-24*
2.4
-1.1
-0.9
2.5
-1.1
2.5
-1.1
-0.8
2.6
-0.9
2.4
-1.3
-1.0
2.6
-1.3
2.5
-1.2
-0.8
2.7
-1.2
2.4
-0.7
-0.8
2.6
-0.7
2.5
-0.6
-0.7
2.7
-0.6
Erythema
B*
C-12*
C-24*
2.4
-1.0
-1.1
2.7
-0.8
2.2
-0.9
-0.7
2.5
-0.8
2.4
-1.0
-0.9
2.8
-1.1
2.3
-1.0
-0.8
2.5
-0.8
2.3
-0.6
-0.7
2.7
-0.5
2.2
-0.5
-0.6
2.5
-0.5
Global improvement over baseline at the end of 12 weeks of treatment in these two trials is shown in Table 2.
Table 2. Global Improvement over Baseline after Twelve Weeks of Treatment in Two Controlled Clinical Trials for Psoriasis
Tazarotene Gel, 0.05%
Tazarotene Gel, 0.1%
Vehicle Gel
N=81
N=93
N=79
N=69
N=84
N=91
100% improvement
2 (2%)
1 (1%)
0
0
1 (1%)
0
≥75% improvement
23 (28%)
17 (18%)
30 (38%)
17 (25%)
10 (12%)
9 (10%)
≥50% improvement
42 (52%)
39 (42%)
51 (65%)
36 (52%)
28 (33%)
21 (23%)
1-49% improvement
21 (26%)
32 (34%)
18 (23%)
23 (33%)
27 (32%)
32 (35%)
No change or worse
18 (22%)
22 (24%)
10 (13%)
10 (14%)
29 (35%)
38 (42%)
The 0.1% gel was more effective than the 0.05% gel, but the 0.05% gel was associated with less local irritation than the 0.1% gel [see Adverse Reactions (6.1)].
Acne: In two large vehicle-controlled trials, tazarotene gel, 0.1% applied once daily was significantly more effective than vehicle in the treatment of facial acne vulgaris of mild to moderate severity. Percent reductions in lesion counts after treatment for 12 weeks in these two trials are shown in Table 3.
Table 3. Reduction in Lesion Counts after Twelve Weeks of Treatment in Two Controlled Clinical Trials for Acne
Tazarotene Gel, 0.1%
Vehicle Gel
N=150
N=149
N=148
N=149
Noninflammatory
lesions
55%
43%
35%
27%
Inflammatory lesions
42%
47%
30%
28%
Total lesions
52%
45%
33%
27%
Global improvement over baseline at the end of 12 weeks of treatment in these two trials is shown in Table 4.
Table 4. Global Improvement over Baseline after Twelve Weeks of Treatment in Two Controlled Clinical Trials for Acne
Tazarotene Gel, 0.1%
Vehicle Gel
N=105
N=117
N=117
N=110
100% improvement
1 (1%)
0
0
0
≥75% improvement
40 (38%)
21 (18%)
23 (20%)
11 (10%)
≥50% improvement
71 (68%)
56 (48%)
47 (40%)
32 (29%)
1-49% improvement
23 (22%)
49 (42%)
48 (41%)
46 (42%)
No change or worse
11 (10%)
12 (10%)
22 (19%)
32 (29%)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Tazarotene gel is a translucent, aqueous gel, available in concentrations of 0.05% and 0.1%. It is available in a collapsible aluminum tube with a tamper-evident aluminum membrane over the opening and a white propylene screw cap, in 30 g and 100 g sizes.
Tazarotene gel, 0.05% 30 g NDC: 73473-309-30
Tazarotene gel, 0.05% 100 g NDC: 73473-309-10
Tazarotene gel, 0.1% 30 g NDC: 73473-310-30
Tazarotene gel, 0.1% 100 g NDC: 73473-310-10Storage: Store at 20℃ to 25℃ (68℉ to 77℉). Excursions permitted from 15℃ to 30℃ (59℉ to 86℉).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Embryofetal Toxicity
Inform females of reproductive potential of the potential risk to a fetus. Advise these patients to use effective contraception during treatment with tazarotene gel. Advise patients to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1, 8.3)].Photosensitivity and Risk of Sunburn
Advise patients to avoid excessive sun exposure and to use of sunscreens and protective measures (hat, visor). Advise patients to avoid using tazarotene gel if also taking other medicines may increase sensitivity to sunlight.Important Administration Instructions
Advise the patient of the following:
1. For the patient with psoriasis, apply tazarotene gel only to psoriasis skin lesions, avoiding uninvolved skin.
2. If undue irritation (redness, peeling, or discomfort) occurs, reduce frequency of application or temporarily interrupt treatment. Treatment may be resumed once irritation subsides [see Dosage and Adminstration (2.1)].
3. Moisturizers may be used as frequently as desired.
4. Patients with psoriasis may use a cream or lotion to soften or moisten skin at least 1 hour before applying tazarotene gel.
5. Avoid contact with the eyes. If tazarotene gel gets in or near eyes, rinse thoroughly with water. Seek medical attention if eye irritation continues.
6. Tazarotene gel is for topical use only. Do not apply to eyes, mouth, or other mucous membrane. Not for ophthalmic, oral, or intravaginal use.
7. Wash hands thoroughly after applying tazarotene gel.Manufactured for:
Solaris Pharma Corporation
Bridgewater, NJ 08807Pharmacist: Please cut or tear at dotted line and provide this patient package insert to your customer
This Patient Information has been approved by the U.S. Food and Drug Administration
- SPL PATIENT PACKAGE INSERT SECTION
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL
100 g Tube Carton Label for Tazarotene Gel, 0.05%
NDC: 73473-309-10
Tazarotene Gel, 0.05%
For Dermatologic Use Only
Not for Ophthalmic Use
Rx Only100 gram

100 g Tube Carton Label for Tazarotene Gel, 0.1%
NDC: 73473-310-10
Tazarotene Gel, 0.1%
For Dermatologic Use Only
Not for Ophthalmic Use
Rx Only100 gram

-
INGREDIENTS AND APPEARANCE
TAZAROTENE
tazarotene gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73473-309 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TAZAROTENE (UNII: 81BDR9Y8PS) (TAZAROTENE - UNII:81BDR9Y8PS) TAZAROTENE 0.5 mg in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73473-309-30 1 in 1 CARTON 01/03/2025 1 30 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 73473-309-10 1 in 1 CARTON 01/03/2025 2 100 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213644 01/03/2025 TAZAROTENE
tazarotene gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73473-310 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TAZAROTENE (UNII: 81BDR9Y8PS) (TAZAROTENE - UNII:81BDR9Y8PS) TAZAROTENE 1 mg in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73473-310-30 1 in 1 CARTON 01/03/2025 1 30 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 73473-310-10 1 in 1 CARTON 01/03/2025 2 100 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213644 01/03/2025 Labeler - Solaris Pharma Corporation (079904672)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.