BAQSIMI- glucagon powder
Baqsimi by
Drug Labeling and Warnings
Baqsimi by is a Prescription medication manufactured, distributed, or labeled by Amphastar Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BAQSIMI safely and effectively. See full prescribing information for BAQSIMI.
BAQSIMI (glucagon) nasal powder
Initial U.S. Approval: 1960RECENT MAJOR CHANGES
Indications and Usage (1)……………………………………….03/2025
INDICATIONS AND USAGE
BAQSIMI® is an antihypoglycemic agent indicated for the treatment of severe hypoglycemia in adults and pediatric patients aged 1 year and older with diabetes. (1)
DOSAGE AND ADMINISTRATION
- BAQSIMI is for intranasal use only. (2.1)
- The recommended dose of BAQSIMI is 3 mg administered as one actuation of the intranasal device into one nostril. (2.2)
- Administer BAQSIMI according to the printed instructions on the shrink-wrapped tube label and the Instructions for Use. (2.1)
- Administer the dose by inserting the tip into one nostril and pressing the device plunger all the way in until the green line is no longer showing. The dose does not need to be inhaled. (2.1)
- Call for emergency assistance immediately after administering the dose. (2.1)
- When the patient responds to treatment, give oral carbohydrates. (2.1)
- Do not attempt to reuse BAQSIMI. Each BAQSIMI device contains one dose of glucagon and cannot be reused. Discard any unused portion. (2.1)
- If there has been no response after 15 minutes, an additional 3 mg dose may be administered while waiting for emergency assistance. (2.2)
DOSAGE FORMS AND STRENGTHS
Nasal powder: intranasal device containing one dose of glucagon 3 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Substantial Increase in Blood Pressure in Patients with Pheochromocytoma: Contraindicated in patients with pheochromocytoma because BAQSIMI may stimulate the release of catecholamines from the tumor. (5.1)
- Hypoglycemia in Patients with Insulinoma: In patients with insulinoma, administration may produce an initial increase in blood glucose; however, BAQSIMI may stimulate exaggerated insulin release from an insulinoma and cause hypoglycemia. If a patient develops symptoms of hypoglycemia after a dose of BAQSIMI, give glucose orally or intravenously. (5.2)
- Serious Hypersensitivity Reactions: Serious hypersensitivity reactions have been reported and include generalized rash, and in some cases anaphylactic shock with breathing difficulties, and hypotension. (5.3)
- Lack of Efficacy in Patients with Decreased Hepatic Glycogen: BAQSIMI is effective in treating hypoglycemia only if sufficient hepatic glycogen is present. Patients in states of starvation, with adrenal insufficiency or chronic hypoglycemia may not have adequate levels of hepatic glycogen for BAQSIMI to be effective. Patients with these conditions should be treated with glucose. (5.4)
ADVERSE REACTIONS
Most common (≥10%) adverse reactions associated with BAQSIMI are nausea, vomiting, headache, upper respiratory tract irritation (i.e., rhinorrhea, nasal discomfort, nasal congestion, cough, and epistaxis), watery eyes, redness of eyes, itchy nose, throat and eyes. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amphastar Pharmaceuticals, Inc. at 1-800-423-4136 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Beta-blockers: Patients taking beta-blockers may have a transient increase in pulse and blood pressure. (7.1)
- Indomethacin: In patients taking indomethacin BAQSIMI may lose its ability to raise glucose or may produce hypoglycemia. (7.2)
- Warfarin: BAQSIMI may increase the anticoagulant effect of warfarin. (7.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosage in Adults and Pediatric Patients Aged 1 Year and Older
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Substantial Increase in Blood Pressure in Patients with Pheochromocytoma
5.2 Hypoglycemia in Patients with Insulinoma
5.3 Serious Hypersensitivity Reactions
5.4 Lack of Efficacy in Patients with Decreased Hepatic Glycogen
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Beta-blockers
7.2 Indomethacin
7.3 Warfarin
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adult Patients with Type 1 or Type 2 Diabetes Mellitus
14.2 Pediatric Patients Aged 1 to less than 17 Years with Type 1 Diabetes Mellitus
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
BAQSIMI is for intranasal use only.
Instruct patients and their caregivers on the signs and symptoms of severe hypoglycemia. Because severe hypoglycemia requires help of others to recover, instruct the patient to inform those around them about BAQSIMI and its Instructions for Use. Administer BAQSIMI as soon as possible when severe hypoglycemia is recognized.
Instruct the patient or caregiver to read the Instructions for Use at the time they receive a prescription for BAQSIMI. Emphasize the following instructions to the patient or caregiver:
- Do not push the plunger or test the device prior to administration.
- Administer BAQSIMI according to the printed instructions on the shrink-wrapped tube label and the Instructions for Use.
- Administer the dose by inserting the tip into one nostril and pressing the device plunger all the way in until the green line is no longer showing. The dose does not need to be inhaled.
- Call for emergency assistance immediately after administering the dose.
- If there has been no response after 15 minutes, an additional dose of BAQSIMI may be administered while waiting for emergency assistance.
- When the patient responds to treatment, give oral carbohydrates to restore the liver glycogen and prevent recurrence of hypoglycemia.
- Do not attempt to reuse BAQSIMI. Each BAQSIMI device contains one dose of glucagon and cannot be reused. Discard any unused portion.
2.2 Dosage in Adults and Pediatric Patients Aged 1 Year and Older
The recommended dose of BAQSIMI is 3 mg administered as one actuation of the intranasal device into one nostril.
If there has been no response after 15 minutes, an additional 3 mg dose of BAQSIMI from a new device may be administered while waiting for emergency assistance.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
BAQSIMI is contraindicated in patients with:
- Pheochromocytoma because of the risk of substantial increase in blood pressure [see Warnings and Precautions (5.1)]
- Insulinoma because of the risk of hypoglycemia [see Warnings and Precautions (5.2)]
- Prior hypersensitivity reaction to glucagon or to any of the excipients in BAQSIMI. Allergic reactions have been reported with glucagon and include anaphylactic shock with breathing difficulties and hypotension [see Warnings and Precautions (5.3)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Substantial Increase in Blood Pressure in Patients with Pheochromocytoma
BAQSIMI is contraindicated in patients with pheochromocytoma because glucagon may stimulate release of catecholamines from the tumor [see Contraindications (4)]. If the patient develops a substantial increase in blood pressure and a previously undiagnosed pheochromocytoma is suspected, 5 to 10 mg of phentolamine mesylate, administered intravenously, has been shown to be effective in lowering blood pressure.
5.2 Hypoglycemia in Patients with Insulinoma
In patients with insulinoma, administration of glucagon may produce an initial increase in blood glucose; however, BAQSIMI administration may directly or indirectly (through an initial rise in blood glucose) stimulate exaggerated insulin release from an insulinoma and cause hypoglycemia. BAQSIMI is contraindicated in patients with insulinoma [see Contraindications (4)]. If a patient develops symptoms of hypoglycemia after a dose of BAQSIMI, give glucose orally or intravenously.
5.3 Serious Hypersensitivity Reactions
Serious hypersensitivity reactions have been reported with glucagon products, including generalized rash, and in some cases anaphylactic shock with breathing difficulties and hypotension. Discontinue BAQSIMI if symptoms of serious hypersensitivity reactions occur. Advise patients and/or caregivers to seek immediate medical attention if the patient experiences any symptoms of serious hypersensitivity reactions. BAQSIMI is contraindicated in patients with a prior hypersensitivity reaction [see Contraindications (4)].
5.4 Lack of Efficacy in Patients with Decreased Hepatic Glycogen
Patients with insufficient hepatic stores of glycogen may not respond to BAQSIMI for the treatment of severe hypoglycemia [see Clinical Pharmacology (12.2)]. Insufficient hepatic stores of glycogen may be present in conditions such as states of starvation or in patients with adrenal insufficiency or chronic hypoglycemia.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
- Substantial Increase in Blood Pressure in Patients with Pheochromocytoma [see Warnings and Precautions (5.1)].
- Hypoglycemia in Patients with Insulinoma [see Warnings and Precautions (5.2)].
- Serious Hypersensitivity Reactions [see Warnings and Precautions (5.3)].
- Lack of Efficacy in Patients with Decreased Hepatic Glycogen [see Warnings and Precautions (5.4)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of BAQSIMI cannot be directly compared with rates in clinical trials of other drugs and may not reflect the rates observed in practice.
Adverse Reactions in Adult Patients
Two similarly designed comparator-controlled trials, Study 1 and Study 2, evaluated the safety of a single intranasal dose of BAQSIMI compared to a 1 mg dose of intra-muscular glucagon (IMG) in adult patients with diabetes [see Clinical Studies (14.1)].
Table 1 presents adverse reactions that occurred with BAQSIMI at an incidence of ≥2% in a pool of Study 1 and Study 2.
Table 1: Pooled Adverse Reactions (≥2%) in Adult Patients with Type 1 and Type 2 Diabetes in Study 1and Study 2 a Upper Respiratory Tract Irritation: rhinorrhea, nasal discomfort, nasal congestion, cough, and epistaxis.
Adverse Reaction BAQSIMI 3 mg
(N=153)
%Nausea 26 Headache 18 Vomiting 15 Upper Respiratory Tract Irritationa 12 Nasal and ocular symptoms with BAQSIMI were solicited through a patient questionnaire in Study 1 and 2 and these adverse reactions are presented in Table 2.
Table 2: Solicited Nasal and Non-Nasal Adverse Reactions in Adult Patients with Type 1 and Type 2 Diabetes Pooled from Study 1 and 2 a Patients were asked to report whether they have the symptom, as well as severity (mild, moderate, severe) at baseline, and after glucagon administration.
Adverse Reactiona BAQSIMI 3 mg
(n=153)
%Any increase in symptom severitya Watery eyes 59 Nasal congestion 43 Nasal itching 39 Runny nose 35 Redness of eyes 25 Itchy eyes 22 Sneezing 20 Itching of throat 12 Itching of ears 3 Adverse Reactions in Pediatric Patients Aged 1 Year and Above
A single dose of BAQSIMI was compared to weight-based doses of 0.5 mg or 1 mg of IMG in pediatric patients aged 4 to less than 17 years with type 1 diabetes in Study 3 [see Clinical Studies (14.2)].
Table 3 presents adverse reactions that occurred with BAQSIMI in pediatric patients at an incidence of ≥2% in Study 3.
Table 3: Adverse Reactions (≥2%) Occurring in Pediatric Patients Aged 4 to less than 17 Years with Type 1 Diabetes in Study 3 a Upper Respiratory Tract Irritation: nasal discomfort, nasal congestion, sneezing.
Adverse Reaction BAQSIMI 3 mg
(n=36)
%Vomiting 31 Headache 25 Nausea 17 Upper Respiratory Tract Irritationa 17 Nasal and ocular symptoms with BAQSIMI were solicited through a patient questionnaire in pediatric patients in Study 3 and these adverse reactions are presented in Table 4.
Table 4: Solicited Nasal and Non-Nasal Adverse Reactions in Pediatric Patients Aged 4 to less than 17 Years with Type 1 Diabetes in Study 3 a Subjects were asked to report whether they have the symptom, as well as severity (mild, moderate, severe) at baseline, and after glucagon administration.
Adverse Reactiona BAQSIMI 3 mg
(n=36)
%Any increase in symptom severitya Watery eyes 47 Nasal congestion 42 Nasal itching 28 Runny nose 25 Sneezing 19 Itchy eyes 17 Redness of eyes 14 Itching of throat 3 Itching of ears 3 The safety of a single 3 mg intranasal dose of BAQSIMI was assessed in an open-label study of 7 pediatric patients aged 1 to less than 4 years with type 1 diabetes mellitus [see Clinical Studies (14.2)]. The safety profile observed in this trial in pediatric patients was comparable to that observed in adults and pediatric patients aged 4 to less than 17 years [see Clinical Pharmacology (12.2, 12.3)].
Other Adverse Reactions in Adult and Pediatric Patients
Other observed adverse reactions with BAQSIMI-treated patients across clinical trials were, dysgeusia, pruritus, tachycardia, hypertension, and additional upper respiratory tract irritation events (nasal pruritus, throat irritation, and parosmia).
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from case reports and a small number of observational studies with glucagon use in pregnant women over decades of use have not identified a drug associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Multiple small studies have demonstrated a lack of transfer of pancreatic glucagon across the human placental barrier during early gestation. In a rat reproduction study, no embryofetal toxicity was observed with glucagon administered by injection during the period of organogenesis at doses representing up to 40 times the human dose, based on body surface area (mg/m2) (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information available on the presence of glucagon in human or animal milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. However, glucagon is a peptide and would be expected to be broken down to its constituent amino acids in the infant's digestive tract and is therefore, unlikely to cause harm to an exposed infant.
8.4 Pediatric Use
The safety and effectiveness of BAQSIMI for the treatment of severe hypoglycemia in patients with diabetes have been established in pediatric patients aged 1 year and older. Use of BAQSIMI for this indication is supported by evidence from an adequate and well-controlled study in adults with type 1 diabetes mellitus [see Clinical Studies (14.1)], a study in 48 pediatric patients aged 4 to less than 17 years with type 1 diabetes mellitus [see Clinical Studies (14.2)], and additional pharmacokinetic and safety data from a study of seven pediatric patients aged 1 to less than 4 years with type 1 diabetes mellitus [see Adverse Reactions (6.1),Clinical Pharmacology (12.2, 12.3), and Clinical Studies (14.2)].
The safety and effectiveness of BAQSIMI have not been established in pediatric patients younger than 1 year of age.
-
10 OVERDOSAGE
If overdosage occurs, the patient may experience nausea, vomiting, inhibition of GI tract motility, increase in blood pressure and pulse rate. In case of suspected overdosing, serum potassium levels may decrease and should be monitored and corrected if needed. If the patient develops a dramatic increase in blood pressure, phentolamine mesylate has been shown to be effective in lowering blood pressure for the short time that control would be needed. In the event of an overdose of BAQSIMI, consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendation.
-
11 DESCRIPTION
BAQSIMI contains glucagon, an antihypoglycemic agent used to treat severe hypoglycemia. Glucagon is a single-chain polypeptide containing 29 amino acid residues and has a molecular weight of 3483, and is identical to human glucagon.
Its molecular formula is C153H225N43O49S, with the following molecular structure:
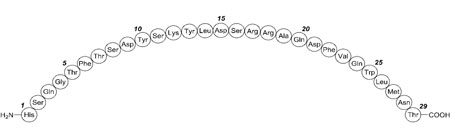
BAQSIMI is a preservative-free, white powder for intranasal administration in an intranasal device containing one dose of 3 mg glucagon. BAQSIMI contains glucagon as the active ingredient and betadex, and dodecylphosphocholine as the excipients.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Glucagon increases blood glucose concentration by activating hepatic glucagon receptors, thereby stimulating glycogen breakdown and release of glucose from the liver. Hepatic stores of glycogen are necessary for glucagon to produce an antihypoglycemic effect.
12.2 Pharmacodynamics
After administration of BAQSIMI in adult patients with diabetes, the mean maximum glucose increase from baseline was 140 mg/dL (Figure 1).
In pediatric patients aged 1 to less than 17 years with type 1 diabetes, the mean maximum glucose increase from baseline was 132 mg/dL (1 to less than 4 years), 138 mg/dL (4 to less than 8 years), 133 mg/dL (8 to less than 12 years), and 102 mg/dL (12 to less than 17 years) (Figure 2).
Sex and body weight had no clinically meaningful effects on the pharmacodynamics of BAQSIMI.
Common cold with nasal congestion tested with or without use of decongestant did not impact pharmacodynamics of BAQSIMI.
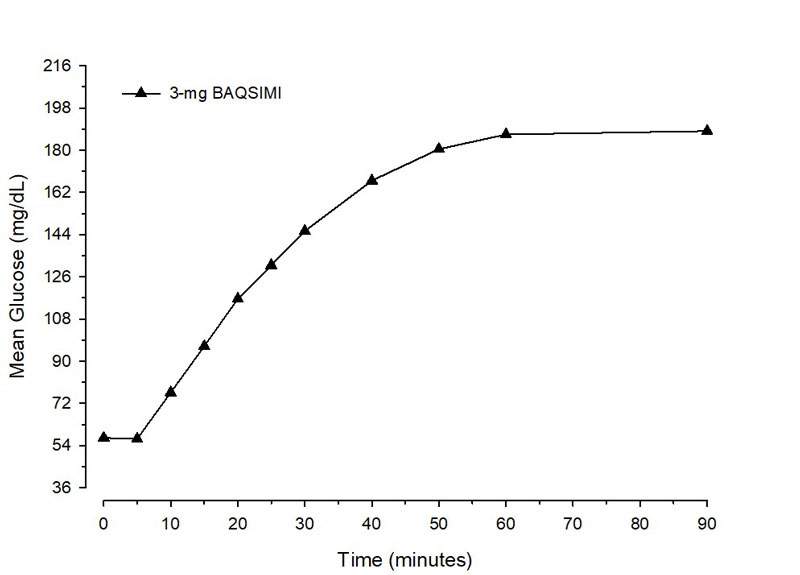
Figure 1 Mean glucose concentration over time after glucagon dose in adult Type 1 Diabetes patientswith insulin-induced hypoglycemia.
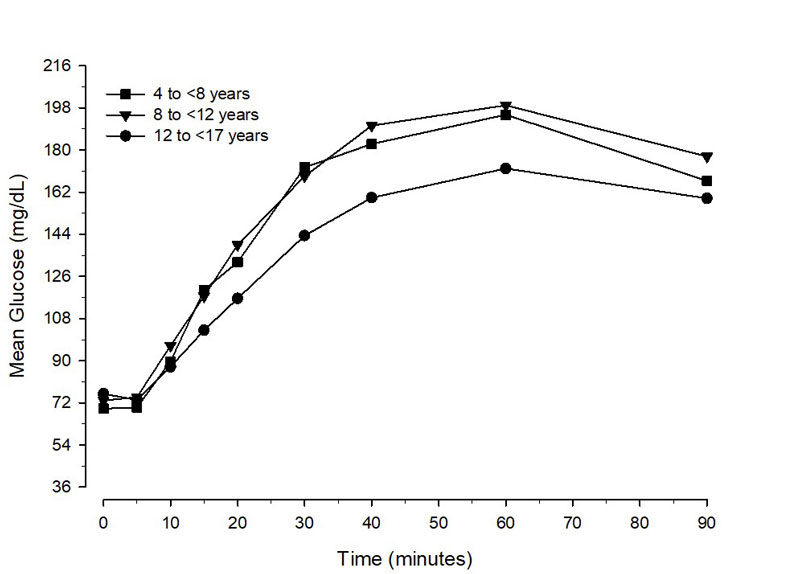
Figure 2 Mean glucose concentration over time in pediatric Type 1 Diabetes patients administered BAQSIMI
12.3 Pharmacokinetics
Absorption
Glucagon absorption via the intranasal route, achieved mean peak plasma levels of 6130 pg/mL at around 15 minutes.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of anti-drug antibodies in other studies, including those of glucagon or of other glucagon products.
In 3 clinical trials, 3/124 (2%) of BAQSIMI-treated patients had treatment-emergent ADAs as detected by an affinity capture elution (ACE) ligand-binding immunogenicity assay. No neutralizing antibodies were detected. Because of the low occurrence of ADA, the effect of these
antibodies on the pharmacokinetics, pharmacodynamics, and/or effectiveness of BAQSIMI is unknown. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies in animals to evaluate carcinogenic potential have not been performed. Recombinant glucagon was positive in the bacterial Ames assay. It was determined that an increase in colony counts was related to technical difficulties in running this assay with peptides. Studies in rats have shown that glucagon does not cause impaired fertility.
-
14 CLINICAL STUDIES
14.1 Adult Patients with Type 1 or Type 2 Diabetes Mellitus
Study 1 (NCT03339453) was a randomized, multicenter, open-label, 2-period, crossover study in adult patients with type 1 diabetes. The efficacy of a single 3 mg dose of BAQSIMI was compared to a 1 mg dose of intra-muscular glucagon (IMG). Insulin was used to reduce blood glucose levels to <60 mg/dL. Seventy patients were enrolled, with a mean age of 41.7 years and a mean diabetes duration of 20.1 years. Twenty-seven (39%) were female.
The primary efficacy outcome measure was the proportion of patients achieving treatment success, which was defined as either an increase in blood glucose to ≥70 mg/dL or an increase of ≥20 mg/dL from glucose nadir within 30 minutes after receiving study glucagon, without receiving additional actions to increase the blood glucose level. Glucose nadir was defined as the minimum glucose measurement at the time of, or within 10 minutes, following glucagon administration.
The mean nadir blood glucose was 54.5 mg/dL for BAQSIMI and 55.8 mg/dL for IMG. BAQSIMI demonstrated non-inferiority to IMG in reversing insulin-induced hypoglycemia with 100% of BAQSIMI-treated patients and 100% of IMG-treated patients achieving treatment success. The mean time to treatment success was 11.6 and 9.9 minutes in the BAQSIMI and IMG 1 mg treatment groups, respectively.
Table 5: Adult Patients with Type 1 Diabetes Meeting Treatment Success and Other Glucose Criteria in Study 1
a The Efficacy Analysis Population consisted of all patients who received both doses of the Study Drug with evaluable primary outcome.
b Difference calculated as (percentage with success in BAQSIMI) – (percentage with success in IMG).
c 2-sided 95% confidence interval (CI) of paired differences using a Wald-Min correction; non-inferiority margin = -10%.
Type 1 Diabetes
(N=66)aBAQSIMI 3 mg IMG
1 mgTreatment Success – n (%) 66 (100%) 66 (100%) Treatment Difference (2-sided 95% confidence limit)b, c 0% (-2.9%, 2.9%) Glucose criterion met – n (%) (i) ≥70 mg/dL
(ii) Increase by ≥20 mg/dL from nadir
Both (i) and (ii)66 (100%)
66 (100%)
66 (100%)66 (100%)
66 (100%)
66 (100%)Study 2 (NCT01994746) was a randomized, multicenter, open-label, 2-period, crossover study in adult patients with type 1 diabetes or type 2 diabetes. The efficacy of a single 3 mg dose of BAQSIMI was compared to a 1 mg dose of intra-muscular glucagon (IMG). Insulin was used to reduce blood glucose levels to the hypoglycemic range with a target blood glucose nadir of <50 mg/dL.
Study 2 enrolled 83 patients 18 to <65 years of age. The mean age of patients with type 1 diabetes (N=77) was 32.9 years and a mean diabetes duration of 18.1 years, and 45 (58%) patients were female. The mean age of patients with type 2 diabetes (N=6) was 47.8 years, with a mean diabetes duration of 18.8 years, and 4 (67%) patients were female.
The mean nadir blood glucose was 44.2 mg/dL for BAQSIMI and 47.2 mg/dL for IMG. BAQSIMI demonstrated non-inferiority to IMG in reversing insulin-induced hypoglycemia with 98.8% of BAQSIMI-treated patients and 100% of IMG-treated patients achieving treatment success within 30 minutes.
The mean time to treatment success was 15.9 and 12.1 minutes in the BAQSIMI and IMG 1 mg treatment groups, respectively.
Table 6: Adult Patients with Type 1 and Type 2 Diabetes Meeting Treatment Success and Other Glucose Criteria in Study 2 a The Efficacy Analysis Population consisted of all patients who received both doses of the Study Drug with evaluable primary outcome.
b Difference calculated as (percentage with success in BAQSIMI) – (percentage with success in IMG).
c 2-sided 95% confidence interval (CI) of paired differences using a Wald-Min correction; non-inferiority margin = -10%.
d Percentage based on number of patients.
Type 1 and Type 2 Diabetes (N=80)a BAQSIMI
3 mgIMG
1 mgTreatment Success – n (%) 79 (98.8%) 80 (100%) Treatment Difference (2-sided 95% confidence limit) b,c -1.3% (-4.6%, 2.2%) Glucose criterion met – n (%)d (i) ≥70 mg/dL 77 (96%) 79 (99%) (ii) Increase by ≥20 mg/dL from nadir 79 (99%) 80 (100%) Both (i) and (ii) 77 (96%) 79 (99%) 14.2 Pediatric Patients Aged 1 to less than 17 Years with Type 1 Diabetes Mellitus
Study 3 (NCT01997411) was a randomized, multicenter, clinical study that assessed BAQSIMI compared to intra-muscular glucagon (IMG) in pediatric patients aged 4 to less than 17 years with type 1 diabetes. Insulin was used to reduce blood glucose levels, and glucagon was administered after glucose reached <80 mg/dL. Efficacy was assessed based on percentage of patients with a glucose increase of ≥20 mg/dL from glucose nadir within 30 minutes following BAQSIMI administration.
Forty-eight patients were enrolled and received at least one dose of study drug. The mean age in the Young Children cohort (4 to <8 years) was 6.5 years. In the Children cohort (8 to <12 years), mean age was 11.1 years and in the Adolescents cohort (12 to <17 years) mean age was 14.6 years. In all age cohorts, the population was predominantly male and white.
Across all age groups, all (100%) patients in both treatment arms achieved an increase in glucose ≥20 mg/dL from glucose nadir within 20 minutes of glucagon administration. The mean time to reach a glucose increase of ≥20 mg/dL for BAQSIMI and IMG for all age groups is shown in Table 7.
Table 7: Mean Time to Reach Glucose Increase of ≥20 mg/dL from Nadir in Pediatric Patients with Type 1 Diabetes in Study 3 Increase from Nadir Mean Time Post-Glucagon Administration (minutes) Young Children
(4 to <8 years old)Children
(8 to <12 years old)Adolescents
(12 to <17 years old)IMGa
N=6BAQSIMI
3 mg
N=12IMGa
N=6BAQSIMI
3 mg
N=12IMGa
N=12BAQSIMI
3 mg
N=12a 0.5 mg or 1 mg of IMG (based upon body weight)
≥20 mg/dL 10.8 10.8 12.5 11.3 12.5 14.2 Study 4 (NCT04992312) was a phase 1, open-label, multi-center study with a primary objective of assessing the safety and tolerability of a single 3 mg dose of BAQSIMI in pediatric participants aged 1 to less than 4 years with type 1 diabetes mellitus. Patients were recommended to fast overnight before the dosing visit on Day 1, to achieve the target range glucose of 70 to 140 mg/dL (3.9 to 7.8 mmol/L) at baseline. Efficacy was assessed based on the percentage of patients with a glucose increase of ≥20 mg/dL from baseline within 30 minutes following BAQSIMI administration.
Seven patients were enrolled in the study, all received the planned 3 mg dose of BAQSIMI and completed the study. The mean age of the patients enrolled in the study was 2.98 years, with ages ranging from 1.8 to 4 years old. There were 4 males and 3 females enrolled in the study, all who were white.
All (100%) patients achieved an increase in glucose ≥20 mg/dL from baseline within 30 minutes of BAQSIMI administration.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
BAQSIMI is supplied as an intranasal device containing one 3 mg dose of glucagon as a preservative free, white powder.
- BAQSIMI One Pack carton contains 1 intranasal device (NDC: 0548-8351-01)
- BAQSIMI Two Pack carton contains 2 intranasal devices (NDC: 0548-8352-02)
- Store at temperatures up to 86°F (30°C) in the shrink wrapped tube provided.
- Keep BAQSIMI in the shrink wrapped tube until ready to use. If the tube has been opened, BAQSIMI may have been exposed to moisture and may not work as expected.
- Discard BAQSIMI and tube after use.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and family members or caregivers to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Recognition of Severe Hypoglycemia:
Inform patient and family members or caregivers on how to recognize the signs and symptoms of severe hypoglycemia and the risks of prolonged hypoglycemia.
Serious Hypersensitivity Reactions:
Inform patients that serious hypersensitivity reactions can occur with BAQSIMI. Advise patients to seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions [see Warnings and Precautions (5.3)].
Marketed by: Amphastar Pharmaceuticals, Inc. Rancho Cucamonga, CA 91730, USA.
Copyright© 2025, Amphastar Pharmaceuticals, Inc. All rights reserved.
698351AMD
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration
Issued: April, 2025
PATIENT INFORMATION
BAQSIMI® (BAK-see-mee)
(glucagon) nasal powderWhat is BAQSIMI?
BAQSIMI is a prescription medicine used to treat very low blood sugar (severe hypoglycemia) in people with diabetes ages 1 year and above.
It is not known if BAQSIMI is safe and effective in children under 1 year of age.Do not use BAQSIMI if you:
- have a tumor in the gland on top of your kidneys (adrenal gland) called pheochromocytoma.
- have a tumor in your pancreas called insulinoma.
- have had an allergic reaction to glucagon, or any of the ingredients in BAQSIMI. See the end of this Patient Information leaflet for a complete list of ingredients in BAQSIMI.
Before using BAQSIMI, tell your healthcare provider about all of your medical conditions, including if you:
- have adrenal gland problems.
- have a tumor in your pancreas.
- have not had food or water for a long time (prolonged fasting or starvation).
- have low blood sugar that does not go away (chronic hypoglycemia).
- are pregnant or plan to become pregnant. It is not known if BAQSIMI will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if BAQSIMI passes into your breast milk. You and your healthcare provider should decide if you can use BAQSIMI while breastfeeding.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. How should I use BAQSIMI?
- Read the detailed Instructions for Use that comes with BAQSIMI.
- Use BAQSIMI exactly how your healthcare provider tells you to use it.
- Make sure your caregiver and those around you know where you keep your BAQSIMI and how to use BAQSIMI the right way before you need their help.
- BAQSIMI contains only 1 dose of medicine and cannot be reused.
- BAQSIMI should be given in one side of your nose (nostril) but does not need to be inhaled.
- BAQSIMI will work even if you have a cold or are taking cold medicine.
- Act quickly. Having very low blood sugar for a period of time may be harmful.
- After giving BAQSIMI, the caregiver should call for emergency medical help right away.
- When you are able to safely swallow food or drink, your caregiver should give you a fast-acting source of sugar (such as a regular soft drink or fruit juice) and a long-acting source of sugar (such as crackers with cheese or peanut butter).
- If the person does not respond after 15 minutes, another dose of BAQSIMI from a new device may be given, if available while waiting for emergency services.
- Tell your healthcare provider each time you use BAQSIMI.
What are the possible side effects of BAQSIMI?
BAQSIMI may cause serious side effects, including:
- High blood pressure. BAQSIMI can cause high blood pressure in certain people with tumors in their adrenal glands.
- Low blood sugar. BAQSIMI can cause certain people with tumors in their pancreas called insulinomas to have low blood sugar.
- Serious allergic reaction. Call your healthcare provider or get medical help right away if you have a serious allergic reaction including:
- rash
- hives
- cough
- difficulty breathing
- trouble swallowing
- swelling of face, lips, or tongue
- low blood pressure
- feeling dizzy or faint
- fast heartbeat
The most common side effects of BAQSIMI include: - nausea
- vomiting
- headache
- runny nose
- discomfort in your nose
- stuffy nose
- cough
- nose bleed
- watery eyes
- redness in your eyes
- itchy nose, throat, and eyes
These are not all the possible side effects of BAQSIMI. For more information, ask your healthcare provider.
Call your healthcare provider for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.How should I store BAQSIMI?
- Store BAQSIMI at temperatures up to 86ºF (30ºC).
- Keep BAQSIMI in the shrink wrapped tube until you are ready to use it.
- Throw away (discard) BAQSIMI and tube after use. Used BAQSIMI may be placed in household trash.
Keep BAQSIMI and all medicines out of the reach of children. General Information about the safe and effective use of BAQSIMI.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use BAQSIMI for a condition for which it was not prescribed. Do not give BAQSIMI to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about BAQSIMI that is written for health professionals.What are the ingredients in BAQSIMI?
Active Ingredient: glucagon
Inactive Ingredients: betadex and dodecylphosphocholineMarketed by: Amphastar Pharmaceuticals, Inc. Rancho Cucamonga, CA 91730, U.S.A.
www.baqsimi.com
Copyright © 2025, Amphastar Pharmaceuticals, Inc. All rights reserved.For more information, call 1-800-423-4136 or go to the following website: www.baqsimi.com. 698351AMD
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
BAQSIMI®
(glucagon) nasal powder
3 mg
Read the Instructions for Use for BAQSIMI before using it. BAQSIMI is used to treat very low blood sugar (severe hypoglycemia) that will cause you to need help from others. You should make sure you show your caregivers, family and friends where you keep BAQSIMI and explain how to use it by sharing these instructions. They need to know how to use BAQSIMI before an emergency happens.
Tube and Device Parts
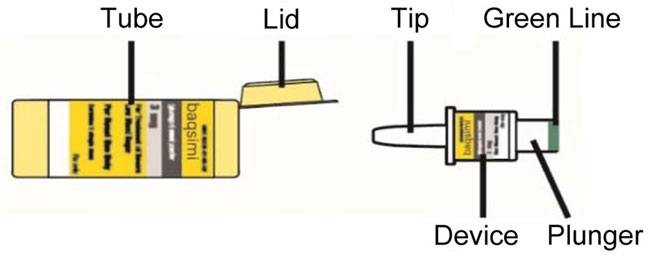
Important Information to Know
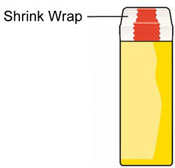
- Do not remove the Shrink Wrap or open the Tube until you are ready to use it.
- If the Tube has been opened, BAQSIMI could be exposed to moisture. This could cause BAQSIMI not to not work as expected.
- Do not push the plunger or test BAQSIMI before you are ready to use it.
- BAQSIMI contains 1 dose of glucagon nasal powder and cannot be reused.
- BAQSIMI is for nasal (nose) use only.
- BAQSIMI will work even if you have a cold or are taking cold medicine.
Preparing the Dose
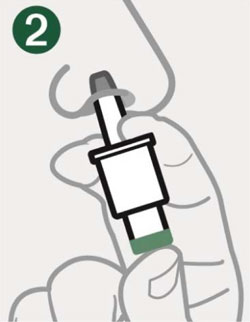
Remove the Shrink Wrap by pulling on red stripe. 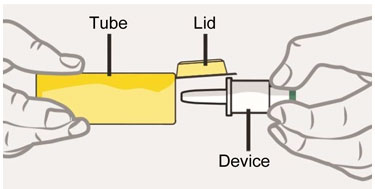
- Open the Lid and remove the Device from the Tube.
Caution: Do not press the Plunger until ready to give the dose.
Giving the Dose
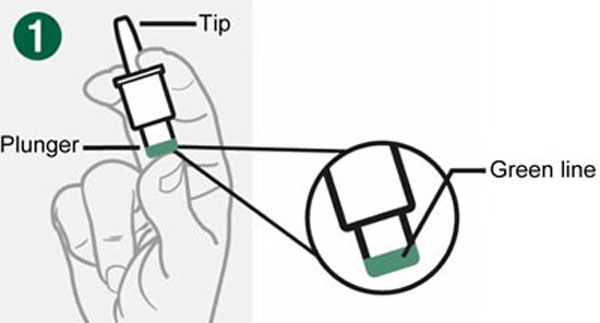
- Hold Device between fingers and thumb.
- Do not push Plunger yet.
- Insert Tip gently into one nostril until finger(s) touch the outside of the nose.
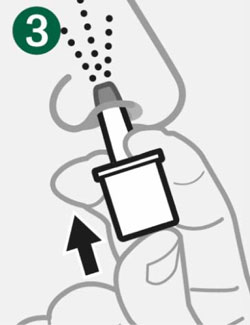
- Push Plunger firmly all the way in.
- Dose is complete when the Green Line disappears.
After giving BAQSIMI
- Call for emergency medical help right away.
- If the person is passed out (unconscious) turn the person on their side.
- Encourage the person to eat as soon as possible. When they can safely swallow, give the person a fast-acting source of sugar such as juice. Then encourage the person to eat a snack such as crackers with cheese or peanut butter.
- If the person does not respond after 15 minutes, another dose may be given, if available.
Storage and Handling
- Do not remove the Shrink Wrap or open the Tube until you are ready to use it.
- Store BAQSIMI in the shrink wrapped Tube at temperatures up to 86º F (30ºC ).
- Throw away (discard) BAQSIMI and Tube after use. Used BAQSIMI may be placed in household trash.
- Caution: Replace the used BAQSIMI right away so you will have a new BAQSIMI in case you need it.
- Replace BAQSIMI before the expiration date printed on the Tube or carton.
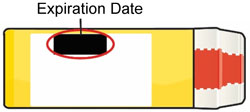
- Keep BAQSIMI and all medicines out of the reach of children.
For Questions or More Information about BAQSIMI
- Call your healthcare provider
- Call Amphastar Pharmaceuticals, Inc. at 1-800-423-4136
- Visit www.baqsimi.com
BAQSIMI is a registered trademark of Amphastar Pharmaceuticals, Inc.
Marketed by: Amphastar Pharmaceuticals, Inc. Rancho Cucamonga, CA 91730, U.S.A
Copyright © 2025, Amphastar Pharmaceuticals, Inc.
BAQSIMI Device meets all applicable requirements defined in ISO 20072 This Instructions for Use has been approved by the U.S. Food and Drug Adminstration
Revised: March, 2025678351AMC/3-25
-
PACKAGE LABEL – Baqsimi 3 mg Nasal Powder One Pack
NDC: 0548-8351-01
baqsimi®
(glucagon) nasal powder
3 mg
For Treatment of Severe Low Blood Sugar
For Nasal Use Only
Contains 1 nasal device.
Keep tube sealed until ready to use.
Rx only
BAQSIMI One Pack®
www.baqsimi.com
568351AMA/5-23
-
PACKAGE LABEL – Baqsimi 3 mg Nasal Powder Two Pack
NDC: 0548-8352-02
baqsimi®
(glucagon) nasal powder
3 mg
For Treatment of Severe Low Blood Sugar
For Nasal Use Only
Contains 2 nasal devices.
Keep tubes sealed until ready to use.
Rx only
BAQSIMI Two Pack®
www.baqsimi.com
528352AMA/5-23
-
INGREDIENTS AND APPEARANCE
BAQSIMI
glucagon powderProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0548-8351 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength glucagon (UNII: 76LA80IG2G) (glucagon - UNII:76LA80IG2G) glucagon 3 mg Inactive Ingredients Ingredient Name Strength Betadex (UNII: JV039JZZ3A) 24 mg Dodecylphosphocholine (UNII: M5CF6282DD) 3 mg Water (UNII: 059QF0KO0R) Acetic Acid (UNII: Q40Q9N063P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0548-8351-01 1 in 1 CARTON 03/15/2024 1 1 in 1 TUBE 1 1 in 1 BOTTLE, UNIT-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210134 07/24/2019 BAQSIMI
glucagon powderProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0548-8352 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength glucagon (UNII: 76LA80IG2G) (glucagon - UNII:76LA80IG2G) glucagon 3 mg Inactive Ingredients Ingredient Name Strength Betadex (UNII: JV039JZZ3A) 24 mg Dodecylphosphocholine (UNII: M5CF6282DD) 3 mg Water (UNII: 059QF0KO0R) Acetic Acid (UNII: Q40Q9N063P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0548-8352-02 2 in 1 CARTON 02/07/2024 1 1 in 1 TUBE 1 1 in 1 BOTTLE, UNIT-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210134 07/24/2019 Labeler - Amphastar Pharmaceuticals, Inc. (024736733)
Trademark Results [Baqsimi]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BAQSIMI 88125097 not registered Live/Pending |
Eli Lilly and Company 2018-09-20 |
 BAQSIMI 87828329 not registered Live/Pending |
Eli Lilly and Company 2018-03-09 |
 BAQSIMI 86648904 5745744 Live/Registered |
ELI LILLY AND COMPANY 2015-06-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.