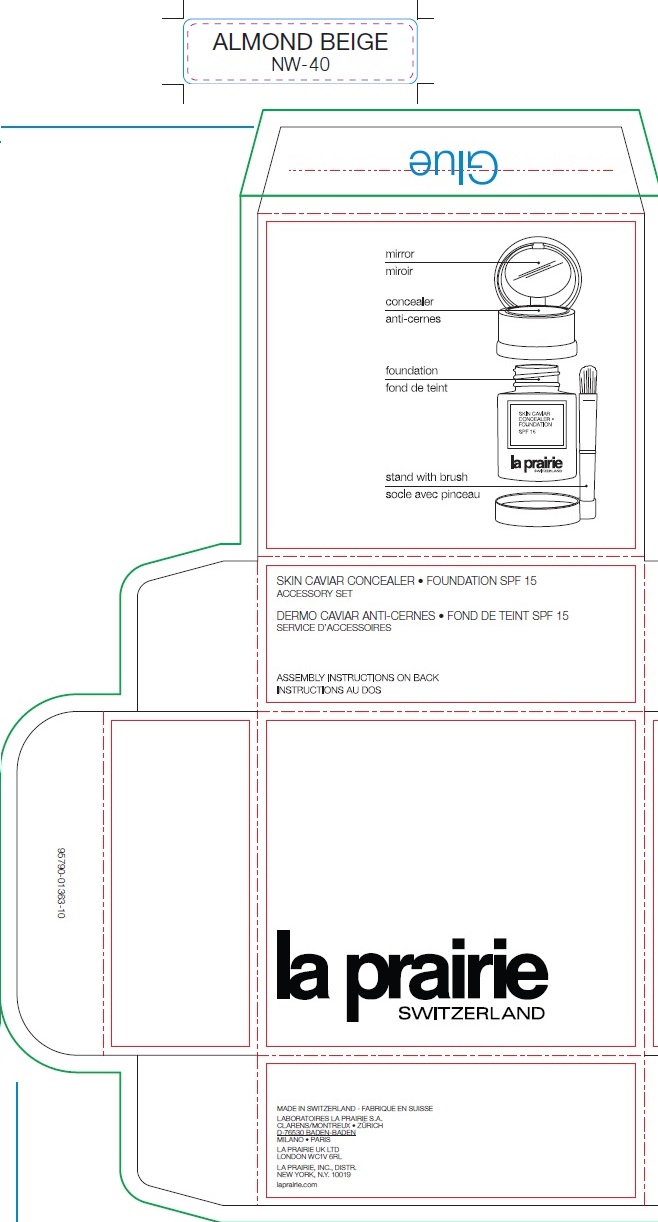
La Prairie Concealer-Foundation Sunscreen SPF 15 - ALMOND BEIGE
La Prairie Concealer-Foundation Sunscreen SPF 15 - ALMOND BEIGE by
Drug Labeling and Warnings
La Prairie Concealer-Foundation Sunscreen SPF 15 - ALMOND BEIGE by is a Otc medication manufactured, distributed, or labeled by La Prairie, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
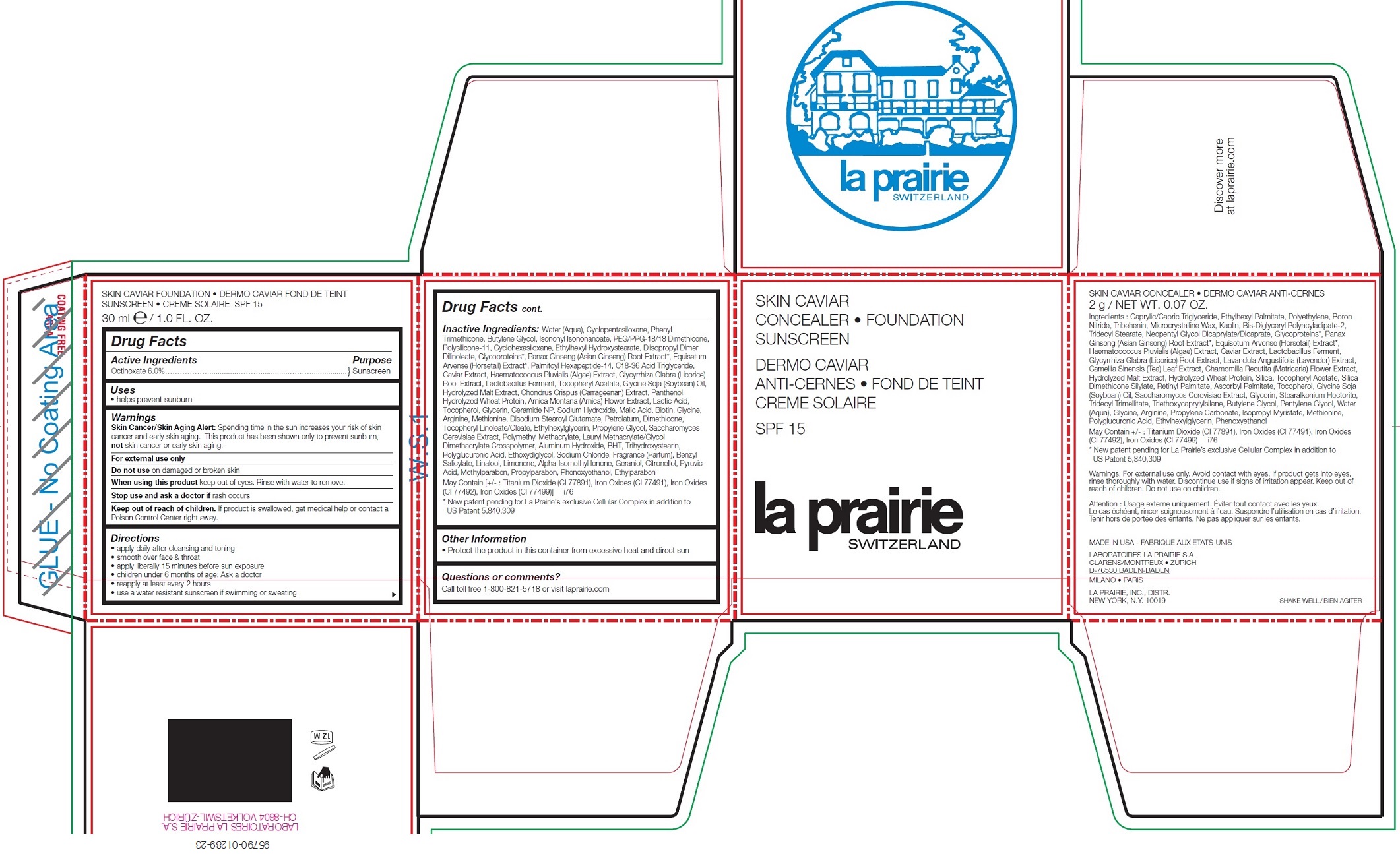
LA PRAIRIE CONCEALER-FOUNDATION SUNSCREEN SPF 15 - ALMOND BEIGE- octinoxate
La Prairie, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
La Prairie Concealer-Foundation Sunscreen SPF 15 - ALMOND BEIGE
Warnings
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early skin aging.
For external use only
Directions
apply daily after cleansing and toning
smooth over face & throat
apply liberally 15 minutes before sun exposure
children under 6 months of age: Ask a doctor
reapply at least every 2 hours
use a water resistant sunscreen if swimming or sweating
Inactive Ingredients:
Water (Aqua), Cyclopentasiloxane, Phenyl Trimethicone, Butylene Glycol, Isononyl Isononanoate, PEG/PPG-18/18 Dimethicone, Polysilicone-11, Cyclohexasiloxane, Ethylhexyl Hydroxystearate, Diisopropyl Dimer Dilinoleate, Glycoproteins*, Panax Ginseng (Asian Ginseng) Root Extract*, Equisetum Arvense (Horsetail) Extract*, Palmitoyl Hexapeptide-14, C18-36 Acid Triglyceride, Caviar Extract, Haematococcus Pluvialis (Algae) Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Lactobacillus Ferment, Tocopheryl Acetate, Glycine Soja (Soybean) Oil, Hydrolyzed Malt Extract, Chondrus Crispus (Carrageenan) Extract, Panthenol, Hydrolyzed Wheat Protein, Arnica Montana (Arnica) Flower Extract, Lactic Acid, Tocopherol, Glycerin, Ceramide NP, Sodium Hydroxide, Malic Acid, Biotin, Glycine, Arginine, Methionine, Disodium Stearoyl Glutamate, Petrolatum, Dimethicone, Tocopheryl Linoleate/Oleate, Ethylhexylglycerin, Propylene Glycol, Saccharomyces Cerevisiae Extract, Polymethyl Methacrylate, Lauryl Methacrylate/Glycol Dimethacrylate Crosspolymer, Aluminum Hydroxide, BHT, Trihydroxystearin, Polyglucuronic Acid, Ethoxydiglycol, Sodium Chloride, Fragrance (Parfum), Benzyl Salicylate, Linalool, Limonene, Alpha-Isomethyl Ionone, Geraniol, Citronellol, Pyruvic Acid, Methylparaben, Propylparaben, Phenoxyethanol, Ethylparaben May Contain [+/- : Titanium Dioxide (CI 77891), Iron Oxides (CI 77491), Iron Oxides (CI 77492), Iron Oxides (CI 77499)] i76
* New patent pending for La Prairie’s exclusive Cellular Complex in addition to US Patent 5,840,309
| LA PRAIRIE CONCEALER-FOUNDATION SUNSCREEN SPF 15 - ALMOND BEIGE
octinoxate kit |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - La Prairie, Inc. (606554996) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.