LIDOCAINE HYDROCHLORIDE AND DEXTROSE- lidocaine hydrochloride injection, solution
Lidocaine Hydrochloride and Dextrose by
Drug Labeling and Warnings
Lidocaine Hydrochloride and Dextrose by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
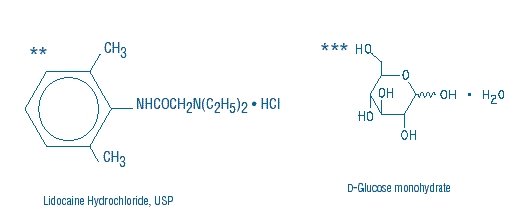
Lidocaine Hydrochloride and 5% Dextrose Injection, USP is a sterile, nonpyrogenic solution prepared from lidocaine hydrochloride and dextrose in water for injection. It contains no antimicrobial agents. Lidocaine hydrochloride is designated chemically as 2-(Diethylamino) - 2', 6' - acetoxylidide monohydrochloride. The solution serves as a cardiac antiarrhythmic agent intended for intravenous use. Composition, osmolarity, pH and caloric content are shown in Table 1. The pH is adjusted with sodium hydroxide.
Table 1 - * Normal physiologic osmolarity range is approximately 280 to 310 mOsmol/L. Administration of substantially hypertonic solutions (≥ 600 mOsmol/L) may cause vein damage.
Composition
*Osmolarity (mOsmol/L)
(calc)pH
Caloric Content
(kcal/L)**Lidocaine Hydrochloride, USP (mg/mL)
***Dextrose Hydrous, USP (g/L)
0.4% Lidocaine Hydrochloride and 5% Dextrose Injection, USP
4
50
282
4.0
(3.0 to 7.0)170
0.8% Lidocaine Hydrochloride and 5% Dextrose Injection, USP
8
50
311
4.0
(3.0 to 7.0)170
This VIAFLEX Plus plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). VIAFLEX Plus on the container indicates the presence of a drug additive in a drug vehicle. The VIAFLEX Plus plastic container system utilizes the same container as the VIAFLEX plastic container system. The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexyl phthalate (DEHP), up to 5 parts per million. However, the safety of the plastic has been confirmed in tests in animals according to USP biological standards for plastic containers as well as by tissue culture toxicity studies.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Lidocaine hydrochloride exerts an antiarrhythmic effect by increasing the electrical stimulation threshold of the ventricle during diastole. In usual therapeutic doses, lidocaine hydrochloride produces no change in myocardial contractility, in systemic arterial pressure, or in absolute refractory period.
Central nervous system adverse reactions become apparent with increasing venous plasma levels above 6.0 μg free base per mL.
Pharmacokinetics
The plasma protein binding of lidocaine is dependent on drug concentration, and the fraction bound decreases with increasing concentration. At concentrations of 1 to 4 micrograms of free base per milliliter 60 to 80 percent of lidocaine is protein bound. Binding is also dependent on the plasma concentration of alpha-1-acid glycoprotein.
Lidocaine crosses the blood-brain and placental barriers, presumably by passive diffusion.
Approximately 90% of lidocaine administered is excreted in the form of various metabolites, and less than 10% is excreted unchanged. Biotransformation includes oxidative N-dealkylation, ring hydroxylation, cleavage of the amide linkage, and conjugation. CYP1A2 and CYP3A4 mediated N-dealkylation, a major pathway of biotransformation, yields the metabolites monoethyl glycine xylidide (MEGX) and glycine xylidide (GX). The pharmacological/toxicological actions of these metabolites are similar to, but less potent than, those of lidocaine. The primary metabolite in urine is a conjugate of 4-hydroxy-2,6-dimethylaniline. The elimination half-life of lidocaine following an intravenous bolus injection is typically 1.5 to 2.0 hours.
Specific Populations
Hepatic Impairment
Because of the rapid rate at which lidocaine is metabolized, any condition that affects liver function may alter lidocaine kinetics. The half-life may be prolonged two-fold or more in patients with liver dysfunction.
Renal Impairment
Mild or moderate renal impairment does not affect lidocaine kinetics; while in patients with severe renal dysfunction, lidocaine clearance is decreased by half and the accumulation of GX increased 1.5-fold.
Lidocaine toxicity is related to systemic blood levels. The decreased clearance and longer half-life of lidocaine should be taken into consideration with prolonged (24 hour) infusions. Constant rate of infusion may result in toxic accumulation of lidocaine.
-
INDICATIONS AND USAGE
Lidocaine hydrochloride administered intravenously is specifically indicated in the acute management of (1) ventricular arrhythmias occurring during cardiac manipulations, such as cardiac surgery and (2) life-threatening arrhythmias which are ventricular in origin, such as occur during acute myocardial infarction.
-
CONTRAINDICATIONS
Hypersensitivity reactions, including anaphylactic reactions, have been reported with lidocaine. Lidocaine hydrochloride is contraindicated in patients with a history of hypersensitivity to local anesthetics of the amide type.
Lidocaine is contraindicated in patients with Stokes-Adams syndrome, Wolff-Parkinson-White syndrome, or with severe degrees of sinoatrial, atrioventricular, or intraventricular block.
-
WARNINGS
Constant monitoring with an electrocardiograph is essential to the administration of lidocaine hydrochloride intravenously. Signs of excessive depression of cardiac conductivity, such as prolongation of the PR interval, widening of the QRS interval and the appearance or aggravation of arrhythmias, should be followed by prompt cessation of the intravenous infusion of this agent. It is mandatory to have emergency resuscitative equipment and drugs immediately available to manage adverse reactions involving cardiovascular, respiratory, or central nervous systems. Central nervous system adverse reactions are associated with venous plasma levels above 6.0 μg free base per mL (see ADVERSE REACTIONS).
Hypersensitivity, including anaphylaxis, has been reported with lidocaine-containing solutions. Stop the infusion immediately if signs of hypersensitivity develop.
Acceleration of ventricular rate may occur in patients with atrial fibrillation or flutter treated with lidocaine.
In patients with sinus bradycardia or incomplete heart block, the administration of lidocaine hydrochloride intravenously for the elimination of ventricular ectopic beats without prior acceleration in heart r ate (e.g., by isoproterenol or by electric pacing) may promote more frequent and serious ventricular arrhythmias or complete heart block (see Contraindications).
Because lidocaine is metabolized mainly in the liver and excreted by the kidneys, patients with renal or hepatic insufficiency may be at increased risk for toxicity.
-
PRECAUTIONS
General:
If malignant hyperthermia develops, discontinue administration immediately and institute therapeutic countermeasures as clinically indicated.
Lidocaine hydrochloride should not be added to blood transfusion assemblies because of the possibilities of pseudoagglutination or hemolysis.
Laboratory Tests:
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Drug Interactions:
Pharmacodynamics Interactions
Digitalis derivatives: Monitor toxicity when lidocaine is used in patients with digitalis toxicity accompanied by supraventricular arrhythmia and/or atrioventricular block (see Contraindications).
When lidocaine is administered with other antiarrhythmic drugs such as amiodarone, phenytoin, procainamide, propranolol or quinidine, the cardiac effects may be additive or antagonistic and toxic effects may be additive.
Pharmacokinetics Interactions
Concomitant treatment with drugs which are inhibitors of CYP1A2 and/or CYP3A4 has the potential to increase lidocaine plasma levels by decreasing lidocaine clearance and thereby prolonging the elimination half-life. Monitor toxicity when administering lidocaine with CYP1A2 and/or CYP3A4 inhibitors.
Concomitant use of lidocaineat steady-state concentrations of the CYP1A2 inhibitor fluvoxamine increases intravenous lidocaine plasma AUC and Cmax by 71% and 22%, and decreases MEGX AUC and Cmax by 54% and 65%. Fluvoxamine decreases the plasma clearance of lidocaine by 41%-60% and prolonged the mean half-life by one hour. Monitor toxicity when coadministering these medications.
Concomitant use of lidocaine with propofol, a hypnotic agent and CYP3A4 inhibitor, may increase lidocaine plasma levels by reducing lidocaine clearance. Monitor toxicity when coadministering lidocaine with propofol.
Concomitant treatment with drugs which are inducers of CYP1A2 and/or CYP3A4 (e.g., phenytoin) has the potential to decrease lidocaine plasma levels and higher doses may be required.
Concomitant use of lidocaine with a weak CYP1A2 and CYP3A4 inhibitor has been reported to increase lidocaine plasma levels by 24% – 75% and may result in toxic accumulation of the drug. Monitor toxicity when coadministering lidocaine with cimetidine.
Beta-adrenergic blockers (e.g. propranolol): Concomitant use of lidocaine with beta-adrenergic blockers may increase lidocaine plasma levels by decreasing hepatic blood flow and thereby decrease lidocaine clearance. Monitor for toxicity when coadministering lidocaine with drugs that decrease hepatic blood flow.
Carcinogenesis, Mutagenesis, and Impairment of Fertility:
Long term animal studies have not been performed to evaluate carcinogenic potential, mutagenic potential or the effect on fertility of lidocaine hydrochloride.
Pregnancy:
Teratogenic Effects:
Reproduction studies have been performed in rats at doses up to five times the maximum human dose and have revealed no significant findings. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, physicians should carefully consider the potential risks and benefits for each specific patient before prescribing lidocaine hydrochloride.
Lidocaine may cross the placental barrier.
Nursing Mothers:
Lidocaine is present in human milk. Published studies have reported a range of lidocaine milk: plasma ratios between 0.4-1.1. Limited data available on lidocaine’s effects on the breastfed child have not revealed a consistent pattern of associated adverse events. The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for lidocaine and any potential adverse effects on the breastfed infant from lidocaine or from the underlying maternal condition.
Geriatric Use
Clinical studies of Lidocaine Hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Systemic reactions of the following types have been reported:
Nervous System Disorders: respiratory depression and arrest; unconsciousness; convulsions; tremors; twitching; vomiting; blurred or double vision; drowsiness; dizziness; light-headedness; tinnitus; sensation of heat, cold or numbness; euphoria; apprehension; agitation; confused state; paresthesia; dysarthria.
Cardiovascular System: cardiovascular arrest; bradycardia which may lead to cardiac arrest; hypotension, Ventricular fibrillation, Ventricular tachycardia, Ventricular arrhythmia, Asystole.
Gastrointestinal Disorders: Hypoesthesia oral, Nausea,
Hematologic Effects: methemoglobinemia.
Psychiatric Disorders: Disorientation
Allergic reactions, including anaphylactic reactions, may occur but are infrequent. There have been no reports of cross sensitivity between lidocaine hydrochloride and procainamide or between lidocaine hydrochloride and quinidine.
-
OVERDOSAGE
Signs and symptoms of overdose may include:
- Central nervous system effects, e.g., coma, loss of consciousness, CNS depression, seizure, tonic-clonic muscle jerks, tremor, nystagmus, tingling of tongue and lips, tinnitus, drowsiness, disorientation, and lightheadedness.
- Cardiorespiratory effects, e.g., cardiovascular collapse and cardiorespiratory arrest (sometimes fatal), respiratory depression and arrest, hypotension, myocardial depression, arrhythmias, including asystole, heart block, ventricular arrhythmias, tachycardia, and bradycardia.
Discontinue lidocaine administration in the event of an overdose.
There is no specific antidote for overdose of lidocaine. The risk of overdose can be minimized by close monitoring during treatment.
Emergency procedures should include appropriate corrective, resuscitative, and other supportive measures (See WARNINGS).
-
DOSAGE AND ADMINISTRATION
Therapy of ventricular arrhythmias is often initiated with a single IV bolus of 1.0 to 1.5 mg/kg at a rate of 25 to 50 mg/min. of lidocaine hydrochloride injection. Following acute treatment by bolus in patients in whom arrhythmias tend to recur and who are incapable of receiving oral antiarrhythmic agents, intravenous infusion of Lidocaine Hydrochloride and 5% Dextrose Injection, USP is administered continuously at the rate of 1 to 4 mg/min (0.020 to 0.050 mg/kg/min in the average 70 kg adult). The 0.4% solution (4 mg/mL) can be given at a rate of 15 to 60 mL/hr (0.25 to 1 mL/min). The 0.8% solution (8 mg/mL) can be given at a rate of 7.5 to 30 mL/hr (0.12 to 0.5 mL/min). Precise dosage regimen is determined by patient characteristics and response.
Infusion rate should be reduced by approximately one-half to compensate for decreased rate of clearance after prolonged infusion (24 hours) (see Clinical Pharmacology). Failure to adjust the rate of infusion in keeping with this altered ability to eliminate lidocaine may result in toxic accumulation of the drug in the patient’s serum.
Intravenous infusions of lidocaine hydrochloride must be administered under constant ECG monitoring to avoid potential overdosage and toxicity. Intravenous infusion should be terminated as soon as the patient’s basic cardiac rhythm appears to be stable or at the earliest signs of toxicity (see OVERDOSAGE). It should rarely be necessary to continue intravenous infusions beyond 24 hours. As soon as possible and when indicated, patients should be changed to an oral antiarrhythmic agent for maintenance therapy.
Caution: When administering lidocaine hydrochloride by continuous infusion, it is advisable to closely monitor the infusion rate. Administer Lidocaine Hydrochloride and 5% Dextrose Injection, USP only with a calibrated infusion device.
Pediatric: Clinical studies to establish pediatric dosing schedules have not been conducted. The usual dosage is a bolus dose of 1 mg/kg followed by an infusion rate of 20 mcg to 50 mcg/kg/min. The bolus dose should be repeated if infusion is not initiated within 15 minutes of the initial bolus dose.
Hepatic impairment is likely to decrease clearance and increase exposure level of lidocaine. Administer lidocaine at lower maintenance infusion rate with close monitoring of toxicity in patients with hepatic impairment.
Renal Impairment: In patients with severe renal impairment (eGFR less than 30 mL/min/1.73 m2), administer lidocaine at lower maintenance infusion rate with close monitoring of toxicity.
Lidocaine is incompatible with the following due to precipitate formation (includes but is not limited to):
- Amphotericin
- Cephazolin sodium
- Phenytoin sodium
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Do not administer unless solution is clear, the seal is intact, and the container is undamaged. Some opacity of the container plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect solution quality or safety. The opacity will diminish gradually. Use of a final filter is recommended during administration of all parenteral solutions, where possible.
Lidocaine must not be infused simultaneously through the same tubing with other medicinal products without first verifying their compatibility.
Set the vent to the close position on a vented intravenous administration set to prevent air embolism.
All injections in VIAFLEX Plus plastic containers are intended for intravenous administration using sterile equipment.
Because dosages of this drug are titrated to response, no additives should be made to Lidocaine Hydrochloride and 5% Dextrose Injection, USP.
-
HOW SUPPLIED
Lidocaine Hydrochloride and 5% Dextrose Injection, USP in VIAFLEX plastic container is available as follows:
Code Size (mL) NDC Product Name 2B0972
250
0338-0409-02
Lidocaine Hydrochloride and 5% Dextrose
2B0973
500
0338-0409-03
Injection, USP (4 mg/mL)
2B0962
250
0338-0411-02
Lidocaine Hydrochloride and 5% Dextrose Injection, USP (8 mg/mL)
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended the product be stored at room temperature (25°C); brief exposure up to 40°C does not adversely affect the product.
- SPL UNCLASSIFIED SECTION
-
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
Container
LOT
EXP
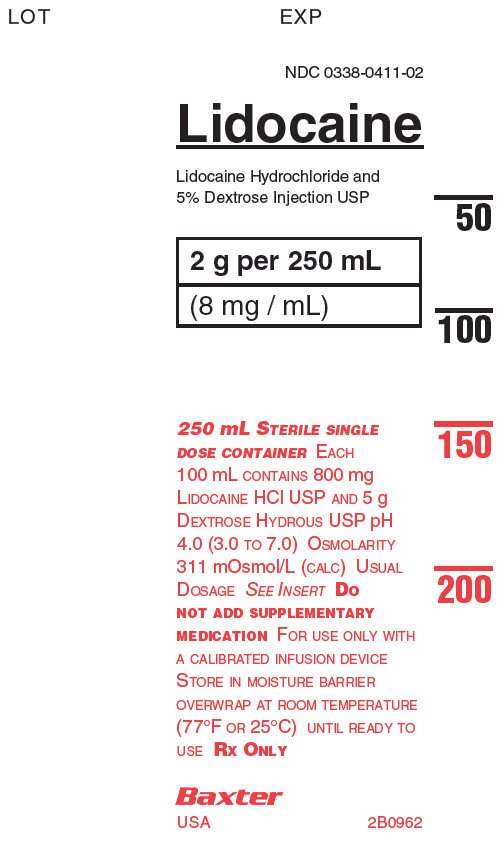
NDC: 0338-0411-02
Lidocaine
Lidocaine Hyrdrochloride and
5% Dextrose Injection USP2g per 250 mL
(8 mg/mL)250 mL STERILE SINGLE
DOSE CONTAINER EACH
100 mL CONTAINS 800 mg
LIDOCAINE HCI USP AND 5 g
DEXTROSE HYDROUS USP pH
4.0 (3.0 TO 7.0) OSMOLARITY
311 mOsmol/L (CALC) USUAL
DOSAGE SEE INSERTDO
NOT ADD SUPPLEMENTARY
MEDICATION FOR USE ONLY WITH
A CALIBRATED INFUSION DEVICE
STORE IN MOISTURE BARRIER
OVERWRAP AT ROOM TEMPERATURE
(77°F OR25°C) UNTIL READY TO
USE RX ONLYBaxter
USA 2B096250
100
150
200Carton
Lot: PXXXXXX Exp: XXX XXXX
QTY: 24-250mL Code: 2B0962NDC: 0338-0411-02
LIDOCAINE HYDROCHLORIDE
AND 5% DEXTROSE INJECTION, USP2g per 250 mL
(17)XXXXX00 (10) PXXXXXX
(01) 50303380411028
01/09/13 12:11:41
Container
LOTEXP
NDC: 0338-0409-03
Lidocaine
Lidocaine Hyrdrochloride and 5% Dextrose Injection USP
2g per 500 mL
(4 mg/mL)500 mL STERILE SINGLE DOSE CONTAINER
EACH 100 mL CONTAINS 400 mg LIDOCAINE HCI
USP AND 5 g DEXTROSE HYDROUS USP
pH 4.0 (3.0 TO 7.0) OSMOLARITY 282
mOsmol/L (CALC) USUAL DOSAGE SEE INSERT
DO NOT ADD SUPPLEMENTARY MEDICATION
FOR USE ONLY WITH A CALIBRATED INFUSION DEVICE
STORE IN MOISTURE BARRIER OVERWRAP AT ROOM
TEMPERATURE (77°F OR25°C) UNTIL READY TO
USE RX ONLYBaxter Logo
USA 2B09731
2
3
4
Carton
Lot: PXXXXXX Exp: XXXXXXX
QTY: 18-500 mL Code: 2B0973NDC: 0338-0409-03
LIDOCAINE HYDROCHLORIDE
AND 5% DEXTROSE INJECTION, USP2g per 500 mL
(17)XXXXX00 (10) PXXXXXX
(01) 50303380409032
-
INGREDIENTS AND APPEARANCE
LIDOCAINE HYDROCHLORIDE AND DEXTROSE
lidocaine hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-0409 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE 4 mg in 1 mL Inactive Ingredients Ingredient Name Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) 50 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0409-03 18 in 1 CARTON 04/22/1981 1 500 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC: 0338-0409-02 24 in 1 CARTON 04/22/1981 2 250 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018461 04/22/1981 LIDOCAINE HYDROCHLORIDE AND DEXTROSE
lidocaine hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-0411 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE 8 mg in 1 mL Inactive Ingredients Ingredient Name Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) 50 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0411-02 24 in 1 CARTON 04/22/1981 1 250 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018461 04/22/1981 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 189326168 ANALYSIS(0338-0409, 0338-0411) , LABEL(0338-0409, 0338-0411) , MANUFACTURE(0338-0409, 0338-0411) , PACK(0338-0409, 0338-0411) , STERILIZE(0338-0409, 0338-0411) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 059140764 ANALYSIS(0338-0409, 0338-0411) , LABEL(0338-0409, 0338-0411) , MANUFACTURE(0338-0409, 0338-0411) , PACK(0338-0409, 0338-0411) , STERILIZE(0338-0409, 0338-0411) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 194684502 ANALYSIS(0338-0409, 0338-0411)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.