DESMOPRESSIN ACETATE spray
Desmopressin Acetate by
Drug Labeling and Warnings
Desmopressin Acetate by is a Prescription medication manufactured, distributed, or labeled by Amring Pharmaceuticals, Inc., Ferring International Center S.A.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DESMOPRESSIN ACETATE NASAL SPRAY safely and effectively. See full prescribing information for DESMOPRESSIN ACETATE NASAL SPRAY.
DESMOPRESSIN ACETATE nasal spray
Initial U.S. Approval: 1978INDICATIONS AND USAGE
Desmopressin Acetate Nasal Spray is a vasopressin analog indicated as antidiuretic replacement therapy in the management of central diabetes insipidus for adults and pediatric patients 4 years of age and older. (1)
Limitations of Use:
Desmopressin Acetate Nasal Spray is not indicated for:
- Treatment of nephrogenic diabetes insipidus (1)
- Treatment of primary nocturnal enuresis (1, 5.1)
- Use in patients with conditions that compromise intranasal route of administration (1, 5.2)
- Use in patients with an impaired level of consciousness (1)
- Use in patients requiring doses less than 10 mcg or doses that are not multiples of 10 mcg (1,3)
DOSAGE AND ADMINISTRATION
- For intranasal use only (2.1)
- Instruct patients to prime pump prior to use (2.1)
- Adults: 10 mcg to 40 mcg daily (either as a single dose or divided into two or three daily doses) (2.2)
- Pediatrics: 10 mcg once daily into one nostril up to 30 mcg once daily (or 30 mcg divided as 20 mcg during the morning and 10 mcg at night) (2.2)
- See the Full Prescribing Information for recommendations for switching between desmopressin acetate formulations (2.3)
DOSAGE FORMS AND STRENGTHS
Nasal Spray: 10 mcg per 0.1 mL spray, available in a 5 mL bottle with spray pump delivering 50 sprays (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Adverse reactions that have been identified in patients administered Desmopressin Acetate Nasal Spray are headache, nasal congestion, rhinitis, nosebleed, sore throat, cough, upper respiratory infections, nausea, flushing, and mild abdominal cramps (6)
To report SUSPECTED ADVERSE REACTIONS, contact Amring Pharmaceuticals Inc. at 1-844-Amring1 (1-844-267-4641) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dosage
2.3 Switching Between Desmopressin Acetate Formulations
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hyponatremia
5.2 Altered Absorption in Patients with Nasal Mucosa Abnormalities
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Other Drugs that may Increase Risk of Hyponatremia
7.2 Other Vasoconstrictors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Desmopressin Acetate Nasal Spray is indicated as antidiuretic replacement therapy in the management of central diabetes insipidus in adults and pediatric patients 4 years of age and older.
Limitations of Use:
Desmopressin Acetate Nasal Spray is not indicated for:
- Treatment of nephrogenic diabetes insipidus,
- Treatment of primary nocturnal enuresis [see Warnings and Precautions (5.1)],
- Use in patients with conditions that compromise the intranasal route of administration (e.g., severe nasal congestion and blockage, nasal mucosa atrophy, severe atrophic rhinitis, recent nasal surgery such as transsphenoidal hypophysectomy) [see Warnings and Precautions (5.2)],
- Use in patients with an impaired level of consciousness,
- Use in patients requiring doses less than 10 mcg or doses that are not multiples of 10 mcg [see Dosage Forms and Strengths (3)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Administer Desmopressin Acetate Nasal Spray by intranasal use only. Instruct patients about appropriate fluid restriction during Desmopressin Acetate Nasal Spray treatment [see Warnings and Precautions (5.1)].
Must prime the spray pump prior to the first use. Instruct patients to:
- Prime pump by pressing down on pump four times (if the spray pump is not used for one week, re-prime the pump by pressing down on the pump once).
- Discard Desmopressin Acetate Nasal Spray after 50 sprays since the amount delivered thereafter may be substantially less than the recommended dosage.
2.2 Recommended Dosage
The use of Desmopressin Acetate Nasal Spray is not indicated for patients who require less than 10 mcg doses or doses that are not multiples of 10 mcg because the spray pump can only deliver doses of 10 mcg [see Indications and Usage (1)]. If other doses are required, use another desmopressin acetate product.
Individualize the dosage of Desmopressin Acetate Nasal Spray for each patient with particular attention in pediatric and elderly patients and adjust according to the diurnal pattern of response to limit nocturia and to ensure fluid intake with respect to urine output is not excessive [see Warnings and Precautions (5.1)]. Monitor continued response to Desmopressin Acetate Nasal Spray by urine volume and osmolality to ensure adequate diuresis to limit the risk of hyponatremia, and include measurements of serum sodium and plasma osmolality as needed.
Adults
The recommended dosage in adults is 10 mcg once daily into one nostril up to 40 mcg once daily (or 40 mcg divided into two or three daily doses). If administered more than once a day, adjust for an adequate diurnal rhythm of urine output.
Pediatric Patients
- For pediatric patients requiring doses less than 10 mcg, Desmopressin Acetate Nasal Spray is not indicated.
- For pediatric patients 4 years of age and older, the recommended starting dosage of Desmopressin Acetate Nasal Spray is 10 mcg once daily into one nostril. The dose can be titrated up to 30 mcg once daily (or 30 mcg divided into two daily doses, typically with 20 mcg given in the morning and 10 mcg given at nighttime). If administered more than once a day, adjust for an adequate diurnal rhythm of urine output.
Because administration of desmopressin acetate can been associated with decreased responsiveness with prolonged use, consider increasing the dosage of Desmopressin Acetate Nasal Spray if patients demonstrate decreased response over a long period of time.
2.3 Switching Between Desmopressin Acetate Formulations
When switching from the desmopressin acetate injection to Desmopressin Acetate Nasal Spray, administer 10 times the amount of desmopressin acetate, rounding down to the nearest 10 mcg.
When switching from the desmopressin acetate tablets to Desmopressin Acetate Nasal Spray individual dose titration is required because intranasal desmopressin is approximately 10 to 40 fold more potent than oral (tablet) desmopressin.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Desmopressin Acetate Nasal Spray is contraindicated in patients with:
- Known hypersensitivity to desmopressin acetate or to any of the components of Desmopressin Acetate Nasal Spray. Severe allergic reactions and anaphylaxis have been reported [see Adverse Reactions (6)].
- Renal impairment defined as estimated creatinine clearance (CLcr) by Cockcroft-Gault equation less than 50 mL/min) [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
- Hyponatremia or a history of hyponatremia [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hyponatremia
Excessive fluid intake when urine output is limited by the antidiuretic effect of desmopressin may lead to water intoxication with hyponatremia. Cases of hyponatremia have been reported from postmarketing experience in patients treated with desmopressin acetate. Unless properly diagnosed and treated, hyponatremia can be fatal.
All patients receiving Desmopressin Acetate Nasal Spray should be observed for the following signs or symptoms associated with hyponatremia: headache, nausea/vomiting, decreased serum sodium, weight gain, restlessness, fatigue, lethargy, disorientation, depressed reflexes, loss appetite, irritability, muscle weakness, muscle spasms or cramps and abnormal mental status such as hallucinations, decreased consciousness, and confusion. Severe symptoms due to an extreme decrease in serum sodium and plasma osmolality may include one or a combination of the following: seizure, coma, and/or respiratory arrest.
In order to decrease the risk of water intoxication with hyponatremia, fluid restriction is recommended. Careful fluid intake restriction is particularly important in pediatric and geriatric patients because these patients are at greater risk of developing hyponatremia [see Use in Specific Populations (8.4, 8.5)]. More frequent monitoring of serum sodium levels is recommended in the following patients: those with conditions associated with fluid and electrolyte imbalance, such as cystic fibrosis, heart failure, renal disorders, habitual or psychogenic polydipsia or those taking concomitant drugs that may cause hyponatremia [see Drug Interactions (7.1)].
Desmopressin Acetate Nasal Spray is not an indicated formulation for the treatment of primary nocturnal enuresis due to a higher risk of hyponatremia and hyponatremic convulsions with the use of the nasal spray formulation compared to desmopressin tablets seen in postmarketing reports [see Indications and Usage (1)].
5.2 Altered Absorption in Patients with Nasal Mucosa Abnormalities
Chronic administration of Desmopressin Acetate Nasal Spray may result in changes to nasal mucosa. Nasal mucosa abnormalities (such as scarring and edema) due to chronic administration, or due to other causes (nasal blockage, nasal mucosal atrophy, severe atrophic rhinitis, recent nasal surgery such as transsphenoidal hypophysectomy) may cause erratic, unreliable absorption. Avoid use of Desmopressin Acetate Nasal Spray in such patients [see Indications and Usage (1)] and consider use of other formulations of desmopressin acetate given by other routes of administration.
-
6 ADVERSE REACTIONS
The following serious reactions are described below and elsewhere in the labeling:
- Hyponatremia [see Warnings and Precautions (5.1)].
- Altered Absorption in Patients with Changes in Nasal Mucosa [see Warnings and Precautions (5.2)].
The following adverse reactions have been identified during post-approval use of desmopressin acetate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
Increase in blood pressure, headache, nasal congestion, rhinitis, nosebleed, sore throat, cough, upper respiratory infections, nausea, flushing, and abdominal cramps.
Water intoxication with hyponatremia
Hyponatremic convulsions associated with concomitant use of the following medications: oxybutinin and imipramine [see Drug Interactions (7.1)].
Severe allergic reactions and anaphylaxis [see Contraindications (4)]
-
7 DRUG INTERACTIONS
7.1 Other Drugs that may Increase Risk of Hyponatremia
The concomitant administration of Desmopressin Acetate Nasal Spray with other drugs that may increase the risk of water intoxication with hyponatremia, (e.g., tricyclic antidepressants, selective serotonin re-uptake inhibitors, chlorpromazine, opiate analgesics, NSAIDs, lamotrigine, oxybutynin and carbamazepine) requires more frequent serum sodium monitoring [see Warnings and Precautions (5.1) and Adverse Reactions (6)].
7.2 Other Vasoconstrictors
Desmopressin acetate can elevate blood pressure. Use of large doses of Desmopressin Acetate Nasal Spray with other vasocontrictors may require a reduction of the Desmopressin Acetate dosage [see Adverse Reactions (6)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Prolonged experience with desmopressin in pregnant women over several decades, based on the available published data and case reports, did not identify a drug associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. In addition, in vitro studies with human placenta demonstrate poor placental transfer of desmopressin. No adverse developmental outcomes were observed in animal reproduction studies with administration of desmopressin during organogenesis to pregnant rats and rabbits at doses approximately <1 and 38 times, respectively, the maximum recommended human dose based on body surface area (mg/m2) (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population are unknown. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Desmopressin acetate at up to 50 ng/kg/day was given by subcutaneous injection to pregnant rats, from gestation day 1 to 20 during the period of early embryonic development and organogenesis without teratogenic effects. Desmopressin acetate at up to 10 mcg/kg/day was given to pregnant rabbits by subcutaneous injection from gestation day 6 to 18 during fetal organogenesis without teratogenic effects. These doses of desmopressin acetate represent approximately <1 times (rat) and 38 times (rabbit) the maximum recommended human dose based on body surface area (mg/m2).
8.2 Lactation
Breastfeeding is not expected to result in clinically relevant exposure of the infant to desmopressin following maternal intranasal administration. Desmopressin is poorly transferred into human breastmilk at negligible amounts (see Data). There is no information on the effects of desmopressin on the breastfed infant or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Desmopressin Acetate Nasal Spray and any potential adverse effects on the breastfed infant from Desmopressin Acetate Nasal Spray or from the underlying maternal condition.
Data
A trial was conducted in six healthy lactating women, at greater than 4 months postpartum, to evaluate intranasal administration of 300 mcg single dose of another desmopressin product (7.5 times the recommended adult dose of Desmopressin Acetate Nasal Spray). Samples of maternal plasma and breastmilk were obtained at 0, 30, 60, 120, 240, 360 and 480 min after the drug administration. At 8 hours after dose intake, the levels in the milk ranged between 4.16 and 101 pg/ml, and the plasma levels ranged between 40 and 242 pg/ml. The total amount of desmopressin present in the milk over the 8 hours ranged between 491 pg and 16 ng, which corresponds to 0.0001 - 0.005% of the administered dose to the breastfeeding mother.
8.4 Pediatric Use
Desmopressin Acetate Nasal Spray is indicated as antidiuretic replacement therapy in the management of central diabetes insipidus in pediatric patients 4 years of age and older. Desmopressin Acetate Nasal Spray is not indicated in pediatric patients less than 4 years of age.
Use of Desmopressin Acetate Nasal Spray in pediatric patients 4 years of age and older is supported by evidence from adults and pediatric patients with central diabetes insipidus. Use in pediatric patients requires careful fluid intake restriction to prevent possible water intoxication with hyponatremia [see Warnings and Precautions (5.1)].
8.5 Geriatric Use
Clinical studies of Desmopressin Acetate Nasal Spray did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at a low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or drug therapy
Because elderly patients are more likely to have renal impairment, care should be taken in dose selection, and monitoring renal function is recommended [see Contraindications (4), Use in Specific Populations (8.6)].
Use of Desmopressin Acetate Nasal Spray in geriatric patients requires careful fluid intake restriction to prevent possible water intoxication with hyponatremia [see Warnings and Precautions (5.1)].
8.6 Renal Impairment
Desmopressin acetate is substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with renal impairment than patients with normal renal function. Desmopressin Acetate Nasal Spray is contraindicated in patients with estimated CLcr by Cockcroft-Gault equation less than 50 mL/min [see Clinical Pharmacology (12.1, 12.3), Contraindications (4)].
-
10 OVERDOSAGE
Signs of desmopressin acetate overdosage may include confusion, drowsiness, continuing headache, problems with passing urine, and rapid weight gain due to fluid retention [see Warnings and Precautions (5.1)]. In case of overdosage, reduce the dosage, decrease the frequency of administration, or discontinue Desmopressin Acetate Nasal Spray. There is no known specific antidote for desmopressin acetate.
-
11 DESCRIPTION
Desmopressin Acetate Nasal Spray is a vasopressin analogue of the natural pituitary hormone 8-arginine vasopressin (ADH), an antidiuretic hormone affecting renal water conservation. It is chemically defined as follows:
Molecular weight: 1183.34
Empirical formula: C46H64N14O12S2C2H4O23H2O

1-(3-mercaptopropionic acid)-8-D-arginine vasopressin monoacetate (salt) trihydrate.
Desmopressin Acetate Nasal Spray is an aqueous solution for intranasal use. Each mL contains:
- Desmopressin acetate 0.1 mg
- Sodium chloride 7.5 mg
- Citric acid monohydrate 1.7 mg
- Disodium phosphate dihydrate 3 mg
- Benzalkonium chloride solution (50%) 0.2 mg
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The antidiuretic effects of desmopressin are mediated by stimulation of vasopressin 2 (V2) receptors, thereby increasing water reabsorption in the kidney, and hence reducing urine production. Desmopressin is a replacement hormone for antidiuretic hormone in the treatment of central diabetes insipidus. The change in structure of arginine vasopressin to desmopressin acetate resulted in a decreased vasopressor action and decreased actions on visceral smooth muscle relative to the enhanced antidiuretic activity, so that clinically effective antidiuretic doses were usually below threshold levels for effects on vascular or visceral smooth muscle.
12.2 Pharmacodynamics
The use of Desmopressin Acetate Nasal Spray in patients with central diabetes insipidus reduces urinary output, increases urine osmolality, and decreases plasma osmolality.
12.3 Pharmacokinetics
Absorption: Desmopressin acetate is absorbed through the nasal mucosa.
Elimination: Desmopressin acetate exhibits a biphasic elimination profile, with half-lives of 7.8 and 75.5 minutes for the initial and terminal phases, respectively.
Specific Populations
Renal Impairment: Desmopressin acetate is mainly excreted in the urine. A pharmacokinetic study was conducted in subjects with normal renal function and patients with mild, moderate, and severe renal impairment (n=24, 6 subjects each group) with a single 2 mcg dose of desmopressin acetate injection (this results in approximately 20 times the exposure of 10 mcg of Desmopressin Acetate Nasal Spray). The terminal half-life was 2.8 hours in subjects with normal renal function, 4.0 hours in mild renal impairment, 6.6 hours in moderate renal impairment and 8.7 hours in severe renal impairment. In patients with mild, moderate and severe renal impairment, mean desmopressin exposure was 1.5 fold, 2.4 fold and 3.6 fold higher, respectively compared to that of subjects with normal renal function [see Contraindications (4), Use in Specific Populations (8.6)].
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Desmopressin Acetate Nasal Spray is available as a 5 mL bottle containing an aqueous solution with the spray pump delivering 50 sprays of 10 mcg (0.1 mL) (NDC: 69918-501-05).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use)
Administration
- Inform caregivers for pediatric patients that administration should be supervised to ensure the patient receives the prescribed dose.
- Inform patients that the pump must be primed prior to first use and again if not used for greater than one week. Inform patients that the Desmopressin Acetate Nasal Spray bottle delivers 50 sprays of 10 mcg each following the initial 4 priming pumps.
- Inform patients to discard any solution remaining after 50 sprays since the amount delivered thereafter may be substantially less than 10 mcg of drug.
- Educate patients about the signs and symptoms of hyponatremia and advise them to contact a healthcare provider if such symptoms occur.
- Discuss downward adjustment of fluid intake and monitoring of urine output with patients.
U.S. Pat. Nos. 5,500,413, 5,596,078, and 5,674,850
Manufactured for:
Amring Pharmaceuticals Inc.
Berwyn, PA 19312
Manufactured by:
Ferring GmbH
Kiel, Germany 24109Origin Germany
Rev. 11/2019
2009055538 -
PATIENT PACKAGE INSERT
This Patient Information has been approved by the Food and Drug Administration 11/2019
-
Instructions for Use
Desmopressin Acetate
Nasal Spray, 10 mcg per 0.1 mL
For Intranasal Use Only
Read these instructions before using Desmopressin Acetate Nasal Spray, and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Children should be helped by an adult when using Desmopressin Acetate Nasal Spray, to make sure the right amount of medicine is used.
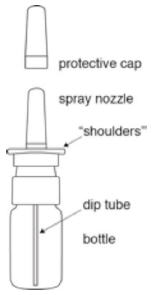
The parts of your Desmopressin Acetate Nasal Spray pump (see Figure A):
Priming your Desmopressin Acetate Nasal Spray:
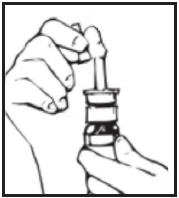
Your Desmopressin Acetate Nasal Spray pump must be primed before you use it for the first time.
- Remove the protective cap (see Figure B).
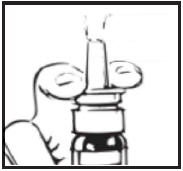
- Press down on the shoulders at the top of your Desmopressin Acetate Nasal Spray pump 4 times. Hold the spray tip away from your face and eyes (see Figure C).
- After your Desmopressin Acetate Nasal Spray pump is primed, it will spray 10 micrograms (1 dose) of medicine each time it is pressed.
Using your Desmopressin Acetate Nasal Spray:
Step 1. Remove the protective cap.
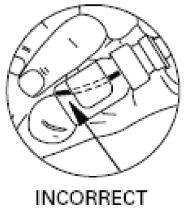
Step 2. To make sure you get the right dose of medicine tilt your Desmopressin Acetate Nasal Spray pump so the dip tube inside the bottle draws the medicine up from the deepest part of the medicine inside the bottle (see Figures D and E).
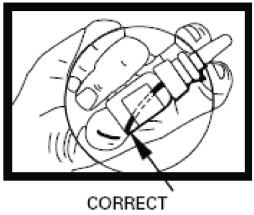
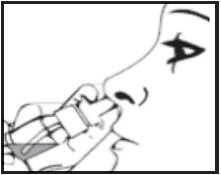
Step 3. Put the spray nozzle tip of your Desmopressin Acetate Nasal Spray into your nostril and press the spray pump 1 time for 1 dose. (see Figure F). If 2 doses are prescribed, spray each nostril 1 time.
Step 4. Put the protective cap back on the spray nozzle tip when you finish using your Desmopressin Acetate Nasal Spray.
Keeping track of your Nasal Sprays:
- Use the check-off chart to help you keep track of your Desmopressin Acetate Nasal Sprays used (see Figure G).
- Keep this chart with your Desmopressin Acetate Nasal Spray or put it someplace where you can easily get it.
- Check off number 1 on the chart with your first dose of Desmopressin Acetate Nasal Spray. Check off the numbers after each use of your Desmopressin Acetate Nasal Spray. If your healthcare provider prescribed a 2-spray dose, then 2 numbers should be checked off.
- Your Desmopressin Acetate Nasal Spray holds 50 sprays with the right amount of medicine in each spray.
- If any medicine is left in your Desmopressin Acetate Nasal Spray after 50 sprays, do not use it. You may not get the right amount of medicine.
- Throw away your Desmopressin Acetate Nasal Spray after 50 sprays.
- Do not count the priming sprays. Your Desmopressin Acetate Nasal Spray has been filled with extra medicine for your priming sprays.
- Do not try to remove any medicine from your Desmopressin Acetate Nasal Spray pump and place it in another bottle.
How should I store Desmopressin Acetate Nasal Spray?
- Store Desmopressin Acetate Nasal Spray at room temperature between 68ºF to 77ºF (20ºC to 25ºC).
- Store Desmopressin Acetate Nasal Spray standing upright.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Amring Pharmaceuticals Inc.
Berwyn, PA 19312

Manufactured by:
Ferring GmbH
Kiel, Germany 24109
Origin Germany
Rev. 11/2019
2009055538
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DESMOPRESSIN ACETATE
desmopressin acetate sprayProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69918-501 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DESMOPRESSIN ACETATE (UNII: XB13HYU18U) (DESMOPRESSIN - UNII:ENR1LLB0FP) DESMOPRESSIN ACETATE 10 ug in 0.1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69918-501-05 1 in 1 CARTON 04/06/2016 1 5 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA AUTHORIZED GENERIC NDA017922 04/06/2016 Labeler - Amring Pharmaceuticals, Inc. (079843051) Registrant - Ferring International Center S.A. (481210362) Establishment Name Address ID/FEI Business Operations Ferring International Center S.A. 481210362 MANUFACTURE(69918-501)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
