VETALOG- triamcinolone acetonide injection, suspension
Vetalog by
Drug Labeling and Warnings
Vetalog by is a Animal medication manufactured, distributed, or labeled by Boehringer Ingelheim Animal Health USA Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Caution:
-
Description:
Vetalog Parenteral is available for veterinary use as a sterile suspension in vials providing 2 mg or 6 mg triamcinolone acetonide per mL with 0.9% (w/v) benzyl alcohol as a preservative, sodium chloride for isotonicity, 0.75% carboxymethylcellulose sodium and 0.04% polysorbate 80. Sodium hydroxide or hydrochloric acid may have been added to adjust the pH. At the time of manufacture, the air in the container is replaced by nitrogen.
- Actions:
-
Rationale For Use: Inflammation and related disorders: Dogs, Cats and Horses:
Injection of Vetalog Parenteral provides rapid relief from pain and reduces inflammation and swelling.
Depending on the nature of the condition, Vetalog Parenteral may be injected intramuscularly, intra-articularly or intrasynovially. The usual pattern of response is improvement of motion and decrease of pain within 24 hours, followed by diminution of swelling.
The extent of return to normal is limited by the degree of irreversible pathologic change present. Triamcinolone acetonide will not reverse permanent pathologic changes.
-
Allergic and Dermatologic Disorders: Dogs and Cats:
Intramuscular or subcutaneous administration of Vetalog Parenteral has been found to provide prompt and prolonged relief in the management of allergic symptoms such as conjunctivitis or reactions to insect bites and in various dermatoses. Inflammation, edema and pruritus are suppressed and discomfort is eased, usually within 24 hours. Since scratching is reduced or eliminated, lesions are permitted to heal more rapidly. In many cases a single injection is sufficient to terminate symptomatology. If necessary, repeat treatments can be administered.
Intralesional administration of Vetalog Parenteral is effective for treatment of dermatological disorders such as moist eczema, frictional acanthosis and other dermatitides in dogs and cats. Inflammation and pruritus are often abated within one to three days. A single intralesional injection is often sufficient to effect remission or elimination of the lesion within a period of one to two weeks.
- Indications:
-
Dosage and Administration:
Prior to withdrawal, the suspension should be inspected for clumping or granular appearance (agglomeration). An agglomerated product results from exposure to freezing temperatures or product being stored in the horizontal position. Product with agglomerates should not be used.
Intramuscular or subcutaneous: Dogs and Cats: The dose is a single injection of 0.05 mg to 0.1 mg triamcinolone acetonide per pound of body weight in inflammatory or allergic disorders and 0.1 mg per pound of body weight in dermatologic disorders. Remission of symptoms, if not permanent, usually lasts 7 to 15 days. After this time, if symptoms recur, the dose may be repeated or oral corticosteroid therapy may be instituted.
Horses: The dose is 0.01 mg to 0.02 mg triamcinolone acetonide per pound of body weight as a single injection; the usual range is 12 mg to 20 mg.
Intralesional: Dogs and Cats: The usual intralesional dosage is 1.2 mg to 1.8 mg triamcinolone acetonide. Injections should be circumscribed around the lesion in various sites to insure adequate distribution of the dose. Injections should be spaced 0.5 cm to 2.5 cm apart, depending on the size of the lesion. The spacing of the dose also reduces pain and/or pressure necrosis.
The dose injected at any one site should not exceed 0.6 mg to minimize local tissue intolerance and atrophy, and should be made well into the cutis to prevent subsequent rupture of the epidermis. When treating dogs and cats with multiple lesions, do not exceed a total dose of 6 mg. Repeat courses of treatment may be administered if necessary.
It is preferable to employ a tuberculin syringe with a small bore needle (23-25 gauge) for accuracy of dose measurement and ease of administration.
Intra-articular and intrasynovial: Dogs, Cats and Horses: The dose for intra-articular or intrasynovial administration is dependent on the size of the joint to be treated and on the severity of symptoms. A single injection of 1 mg to 3 mg triamcinolone acetonide for cats and dogs and 6 mg to 18 mg for horses is recommended. After three or four days, injections may be repeated, depending on the severity of symptoms and the clinical response. If initial results are inadequate or too transient, dosage may be increased, but the recommended dose should not be exceeded.
Routine aseptic preparation of the area should be made prior to all intra-articular injections. A thorough understanding of the pertinent anatomic relationships is essential. The inadvertent administration of the corticosteroid into the soft tissues surrounding a joint is not harmful, but is the most common cause of failure to achieve the desired local results.
Following intra-articular administration, pain and other local symptoms may continue for a short time before effective relief is obtained, but an increase in joint discomfort is rare. A marked increase in pain accompanied by local swelling, further restriction of joint motion, fever and malaise are suggestive of a septic arthritis. If these complications should occur and the diagnosis of sepsis is confirmed, antimicrobial therapy should be instituted immediately and continued until all evidence of infection has disappeared.
- Contraindications:
- Warnings:
-
Usage in Pregnancy:
The safety of most corticosteroid drugs for use during all stages of pregnancy has not been adequately established. However, clinical and experimental data have demonstrated that corticosteroids administered orally or by injection to animals may induce the first stage of parturition if used during the last trimester of pregnancy and may precipitate premature parturition followed by dystocia, fetal death, retained placenta and metritis.
Additionally, corticosteroids administered to dogs, rabbits and rodents during pregnancy have resulted in cleft palate in offspring. Corticosteroids administered to dogs during pregnancy have also resulted in other congenital anomalies including deformed forelegs, phocomelia and anasarca. Therefore, before use of corticosteroids in pregnant animals, the possible benefits to the pregnant animal should be weighed against potential hazards to its developing embryo or fetus.
-
Precautions:
Vetalog Parenteral should not be used to alleviate pain or reduce inflammation arising from infectious states unless concomitant antimicrobial therapy is given.
Because of the anti-inflammatory action of corticosteroids, signs of infection may be hidden and it may be necessary to stop treatment until diagnosis is made.
Overdosage of some glucocorticoids may result in sodium retention, fluid retention, potassium loss and weight gains.
Corticosteroids have been used in the treatment of laminitis; Vetalog Parenteral is not recommended for that use. Cases of laminitis have been reported following the administration of Vetalog Parenteral; the mechanism of that response has not been fully elucidated. Care is necessary when using any corticosteroid in the equine species.
Use of corticosteroids, depending on dose, duration and specific steroid, may result in inhibition of endogenous steroid production following drug withdrawal. In patients presently receiving or recently withdrawn from systemic corticosteroid treatments, therapy with a rapidly acting corticosteroid should be considered in unusually stressful situations.
-
Adverse Reactions:
As with any corticosteroid, polydipsia or polyuria may occur with high dosage or frequent administration of triamcinolone acetonide. The likelihood of their occurrence may be minimized by giving as brief a course of corticosteroid therapy as possible, and by waiting for the reappearance of symptoms before repeating therapy. If polydipsia or polyuria should occur, therapy should be discontinued until these unwanted effects have disappeared; therapy should then be resumed at a lower dosage level.
Other adverse reactions that have occurred with the use of corticosteroids are weight loss, anorexia and diarrhea (occasionally bloody). Anaphylactoid reactions have occasionally been seen following administration.
Intra-articular injection in leg injuries of the horse may produce osseous metaplasia.
Side effects such as serum alkaline phosphatase (SAP) and serum glutamic pyruvic transaminase (SGPT) enzyme elevations have occurred following use of synthetic corticosteroids in dogs.
Cushing’s Syndrome in dogs has been reported in association with prolonged or repeated steroid therapy.
To report suspected adverse reactions, or to obtain a copy of the Material Safety Data Sheet (MSDS), call 1-866-638-2226.
-
How Supplied:
Vetalog Parenteral is supplied for veterinary use in two concentrations and various vial sizes:
NDC: 0010-4703-02 - 2 mg/mL 100 mL (For Horses Only)*
NDC: 0010-4703-01 - 2 mg/mL 25 mL
NDC: 0010-4704-03 - 6 mg/mL 25 mL (For Horses Only)*
NDC: 0010-4704-02 - 6 mg/mL 5 mL
NDC: 0010-4704-01 - 6 mg/mL 3 mL (For Horses Only)
*To limit the number of entries through the stopper, these two vials are for use in HORSES ONLY.
- Storage:
- SPL UNCLASSIFIED SECTION
-
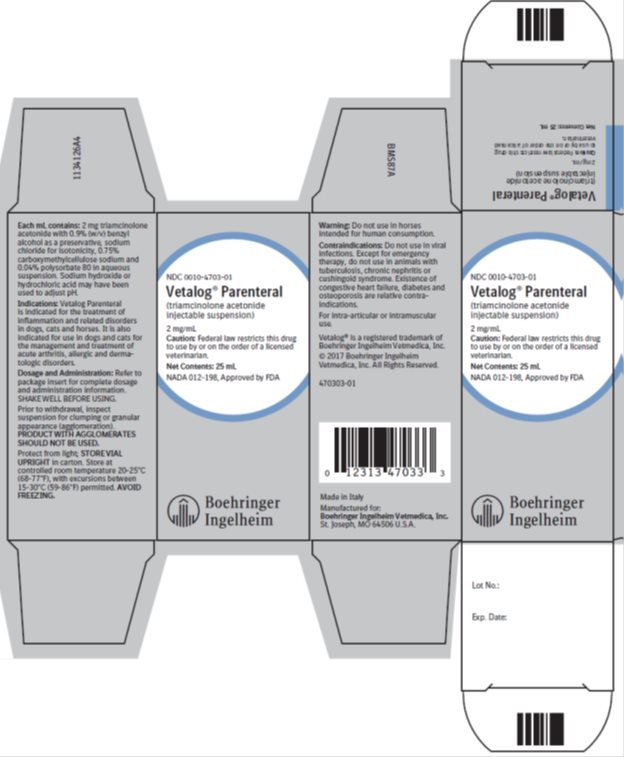
Principal Display Panel – 2 mg/mL, Container label 25 mL
NDC: 0010-4703-01
Vetalog® Parenteral
(triamcinolone acetonide injectable suspension)
2 mg/mL
Caution: Federal law restricts this drug by or on the order of a licensed veterinarian.
Net Contents: 25 mL
-
Principal Display Panel – 2 mg/mL, 25 mL Display Carton
NDC: 0010-4703-01
Vetalog® Parenteral
(triamcinolone acetonide injectable suspension)
2 mg/mL
Caution: Federal law restricts this drug by or on the order of a licensed veterinarian.
Net Contents: 25 mL
-
Principal Display Panel - 6 mg/mL, 25 mL Container label
NDC: 0010-4704-03
Vetalog® Parenteral
(triamcinolone acetonide injectable suspension)
6 mg/mL
Caution: Federal law restricts this drug by or on the order of a licensed veterinarian.
-
Principal Display Panel - 6 mg/mL, 25 mL Display Carton
NDC: 0010-4704-03
Vetalog® Parenteral
(triamcinolone acetonide injectable suspension)
6 mg/mL
Caution: Federal law restricts this drug to use by or on the order of a licesned veterinarian.
Net Contents: 25 mL
NADA 012-198, Approved by FDA
-
INGREDIENTS AND APPEARANCE
VETALOG
triamcinolone acetonide injection, suspensionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0010-4703 Route of Administration INTRAMUSCULAR, INTRA-ARTICULAR, INTRASYNOVIAL, SUBCUTANEOUS, INTRALESIONAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIAMCINOLONE ACETONIDE (UNII: F446C597KA) (TRIAMCINOLONE ACETONIDE - UNII:F446C597KA) TRIAMCINOLONE ACETONIDE 2 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0010-4703-01 1 in 1 CARTON 1 25 mL in 1 VIAL, MULTI-DOSE 2 NDC: 0010-4703-02 1 in 1 CARTON 2 100 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA012198 02/26/2016 VETALOG
triamcinolone acetonide injection, suspensionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0010-4704 Route of Administration INTRAMUSCULAR, INTRA-ARTICULAR, INTRASYNOVIAL, SUBCUTANEOUS, INTRALESIONAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIAMCINOLONE ACETONIDE (UNII: F446C597KA) (TRIAMCINOLONE ACETONIDE - UNII:F446C597KA) TRIAMCINOLONE ACETONIDE 6 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0010-4704-01 1 in 1 CARTON 1 3 mL in 1 VIAL, MULTI-DOSE 2 NDC: 0010-4704-02 1 in 1 CARTON 2 5 mL in 1 VIAL, MULTI-DOSE 3 NDC: 0010-4704-03 1 in 1 CARTON 3 25 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA012198 02/26/2016 Labeler - Boehringer Ingelheim Animal Health USA Inc. (007134091) Registrant - Boehringer Ingelheim Animal Health USA Inc. (007134091)
Trademark Results [Vetalog]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VETALOG 72073596 0690474 Live/Registered |
OLIN MATHIESON CHEMICAL CORPORATION 1959-05-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.