TALZENNA- talazoparib capsule
Talzenna by
Drug Labeling and Warnings
Talzenna by is a Prescription medication manufactured, distributed, or labeled by Pfizer Laboratories Div Pfizer Inc, Viatris Pharmaceuticals LLC, Pharmacia & Upjohn Company LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TALZENNA safely and effectively. See full prescribing information for TALZENNA.
TALZENNA® (talazoparib) capsules, for oral use
Initial U.S. Approval: 2018RECENT MAJOR CHANGES
Dosage and Administration, Dose Modifications for Patients with Renal Impairment (2.4) 3/2020 INDICATIONS AND USAGE
TALZENNA is a poly (ADP-ribose) polymerase (PARP) inhibitor indicated for the treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) HER2-negative locally advanced or metastatic breast cancer. Select patients for therapy based on an FDA-approved companion diagnostic for TALZENNA. (1)
DOSAGE AND ADMINISTRATION
- The recommended dose of TALZENNA is 1 mg taken as a single oral daily dose, with or without food. (2.2)
- Patients should be treated until disease progression or unacceptable toxicity occurs. (2.2)
- For adverse reactions, consider dosing interruption or dose reduction. (2.3)
- For patients with moderate renal impairment (CLcr 30 – 59 mL/min), the recommended dose of TALZENNA is 0.75 mg once daily. (2.4)
- For patients with severe renal impairment (Clcr 15 – 29 mL/min), the recommended dose of TALZENNA is 0.5 mg once daily. (2.4)
DOSAGE FORMS AND STRENGTHS
Capsules: 0.25 mg, 1 mg (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Myelodysplastic Syndrome/Acute Myeloid Leukemia (MDS/AML): MDS/AML has been reported in 2 out of 584 (0.3%) solid tumor patients treated with TALZENNA in clinical studies. Monitor patients for hematological toxicity at baseline and monthly thereafter. Discontinue if MDS/AML is confirmed. (5.1)
- Myelosuppression: TALZENNA may affect hematopoiesis and can cause anemia, neutropenia, and/or thrombocytopenia. (5.2)
- Embryo-Fetal Toxicity: TALZENNA can cause fetal harm. Advise of the potential risk to the fetus and to use effective contraception. (5.3, 8.1, 8.3)
ADVERSE REACTIONS
- Most common (≥20%) adverse reactions of any grade were: Fatigue, anemia, nausea, neutropenia, headache, thrombocytopenia, vomiting, alopecia, diarrhea, decreased appetite. (6.1)
- Most common laboratory abnormalities (≥25%) were: Decreases in hemoglobin, platelets, neutrophils, lymphocytes, leukocytes, and calcium. Increases in glucose, alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Recommended Dosing
2.3 Dose Modifications for Adverse Reactions
2.4 Dose Modifications for Patients with Renal Impairment
2.5 Dose Modifications for Use with P-glycoprotein (P-gp) Inhibitors
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myelodysplastic Syndrome/Acute Myeloid Leukemia
5.2 Myelosuppression
5.3 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on TALZENNA
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
TALZENNA is indicated for the treatment of adult patients with deleterious or suspected deleterious germline breast cancer susceptibility gene (BRCA)-mutated (gBRCAm) human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer. Select patients for therapy based on an FDA-approved companion diagnostic for TALZENNA [see Dosage and Administration (2.1)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
Select patients for the treatment of advanced breast cancer with TALZENNA based on the presence of germline BRCA mutations [see Indications and Usage (1), Clinical Studies (14)]. Information on the FDA-approved test for the detection of BRCA mutations is available at http://www.fda.gov/companiondiagnostics.
2.2 Recommended Dosing
The recommended dose of TALZENNA is 1 mg taken orally once daily, with or without food.
The 0.25 mg capsule is available for dose reduction.
Patients should be treated until disease progression or unacceptable toxicity occurs.
The hard capsules should be swallowed whole and must not be opened or dissolved. If the patient vomits or misses a dose, an additional dose should not be taken. The next prescribed dose should be taken at the usual time.
2.3 Dose Modifications for Adverse Reactions
To manage adverse reactions, consider interruption of treatment with or without dose reduction based on severity and clinical presentation. Recommended dose reductions are indicated in Table 1 and Table 2. Treatment with TALZENNA should be discontinued if more than three dose reductions are required.
Table 1. Dose Reduction Levels for Adverse Reactions Dose Level Dose Recommended starting dose 1 mg (one 1 mg capsule) once daily First dose reduction 0.75 mg (three 0.25 mg capsules) once daily Second dose reduction 0.5 mg (two 0.25 mg capsules) once daily Third dose reduction 0.25 mg (one 0.25 mg capsule) once daily Table 2. Dose Modification and Management
Monitor complete blood counts monthly and as clinically indicated [see Warnings and Precautions (5.2)].
Adverse Reactions Withhold TALZENNA until levels resolve to Resume TALZENNA Hemoglobin <8 g/dL ≥9 g/dL Resume TALZENNA at a reduced dose Platelet count <50,000/μL ≥75,000/μL Neutrophil count <1,000/μL ≥1500/µL Non-hematologic Grade 3 or Grade 4 ≤Grade 1 Consider resuming TALZENNA at a reduced dose or discontinue 2.4 Dose Modifications for Patients with Renal Impairment
For patients with moderate renal impairment (CLcr 30 – 59 mL/min), the recommended dose of TALZENNA is 0.75 mg once daily. For patients with severe renal impairment (CLcr 15 – 29 mL/min), the recommended dose of TALZENNA is 0.5 mg once daily [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
2.5 Dose Modifications for Use with P-glycoprotein (P-gp) Inhibitors
Reduce the TALZENNA dose to 0.75 mg once daily when coadministered with certain P-gp inhibitors. For additional information on interacting P-gp inhibitors, see Drug Interactions (7.1) and Clinical Pharmacology (12.3).
When the P-gp inhibitor is discontinued, increase the TALZENNA dose (after 3–5 half-lives of the P-gp inhibitor) to the dose used prior to the initiation of the P-gp inhibitor [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Myelodysplastic Syndrome/Acute Myeloid Leukemia
Myelodysplastic Syndrome/Acute Myeloid Leukemia (MDS/AML) have been reported in patients who received TALZENNA. Overall, MDS/AML has been reported in 2 out of 584 (0.3%) solid tumor patients treated with TALZENNA in clinical studies. The duration of TALZENNA treatment in these two patients prior to developing MDS/AML was 4 months and 24 months, respectively. Both patients had received previous chemotherapy with platinum agents and/or other DNA damaging agents including radiotherapy.
Do not start TALZENNA until patients have adequately recovered from hematological toxicity caused by previous chemotherapy. Monitor complete blood counts for cytopenia at baseline and monthly thereafter. For prolonged hematological toxicities, interrupt TALZENNA and monitor blood counts weekly until recovery. If the levels have not recovered after 4 weeks, refer the patient to a hematologist for further investigations, including bone marrow analysis and blood sample for cytogenetics. If MDS/AML is confirmed, discontinue TALZENNA.
5.2 Myelosuppression
Myelosuppression consisting of anemia, leukopenia/neutropenia, and/or thrombocytopenia, have been reported in patients treated with TALZENNA [see Adverse Reactions (6)]. Grade ≥3 anemia, neutropenia, and thrombocytopenia were reported, respectively, in 39%, 21%, and 15% of patients receiving TALZENNA. Discontinuation due to anemia, neutropenia, and thrombocytopenia occurred, respectively, in 0.7%, 0.3%, and 0.3% of patients.
Monitor complete blood count for cytopenia at baseline and monthly thereafter. Do not start TALZENNA until patients have adequately recovered from hematological toxicity caused by previous therapy. If this occurs, dose modifications (dosing interruption with or without dose reduction) are recommended [see Dosing Modifications (2.3)].
5.3 Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal data, TALZENNA can cause fetal harm when administered to a pregnant woman. In an animal reproduction study, administration of talazoparib to pregnant rats during the period of organogenesis caused fetal malformations and structural skeletal variations, and embryo-fetal death at exposures that were 0.24 times the area under the concentration-time curve (AUC) in patients receiving the recommended human dose of 1 mg daily. Apprise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for at least 7 months following the last dose of TALZENNA [see Use in Specific Populations (8.1, 8.3), Clinical Pharmacology (12.1)].
Based on findings from genetic toxicity and animal reproduction studies, advise male patients with female partners of reproductive potential or who are pregnant to use effective contraception during treatment and for at least 4 months following the last dose of TALZENNA [see Use in Specific Populations (8.1, 8.3), Nonclinical Toxicology (13.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Myelodysplastic Syndrome/Acute Myeloid Leukemia [see Warnings and Precautions (5.1)]
- Myelosuppression [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Treatment of gBRCAm HER2-negative Locally Advanced or Metastatic Breast Cancer
EMBRACA
The safety of TALZENNA as monotherapy was evaluated in gBRCAm patients with HER2-negative locally advanced or metastatic breast cancer who had previously received no more than 3 lines of chemotherapy for the treatment of locally advanced/metastatic disease. EMBRACA was a randomized, open-label, multi-center study in which 412 patients received either TALZENNA 1 mg once daily (n=286) or a chemotherapy agent (capecitabine, eribulin, gemcitabine, or vinorelbine) of the healthcare provider's choice (n=126) until disease progression or unacceptable toxicity. The median duration of study treatment was 6.1 months in patients who received TALZENNA and 3.9 months in patients who received chemotherapy. Dosing interruptions due to an adverse reaction of any grade occurred in 65% of patients receiving TALZENNA and 50% of those receiving chemotherapy; dose reductions due to any cause occurred in 53% of TALZENNA patients and 40% of chemotherapy patients. Permanent discontinuation due to adverse reactions occurred in 5% of TALZENNA patients and 6% chemotherapy patients.
Table 3 and Table 4 summarize the most common adverse reactions and laboratory abnormalities, respectively, in patients treated with TALZENNA or chemotherapy in the EMBRACA study.
Table 3. Adverse Reactions* (in ≥20% of Patients Receiving TALZENNA) in EMBRACA TALZENNA
N=286 (%)Chemotherapy
N=126 (%)Adverse Reactions Grades 1–4 Grade 3 Grade 4 Grades 1–4 Grade 3 Grade 4 Abbreviations: AR=adverse reaction; CTCAE=Common Terminology Criteria for Adverse Events; NCI=National Cancer Institute; N=number of patients. - * Graded according to NCI CTCAE 4.03.
- † Includes anemia, hematocrit decreased, hemoglobin decreased, and red blood cell count decreased.
- ‡ Includes febrile neutropenia, neutropenia and neutrophil count decreased.
- § Includes thrombocytopenia and platelet count decreased.
- ¶ For TALZENNA, Grade 1 in 23%, and Grade 2 in 2%. For the chemotherapy arm, Grade 1 in 20%, and Grade 2 in 8%.
- # Includes fatigue and asthenia.
Blood and lymphatic system disorders Anemia† 53 38 1 18 4 1 Neutropenia‡ 35 18 3 43 20 16 Thrombocytopenia§ 27 11 4 7 2 0 Metabolism and nutrition disorders Decreased appetite 21 <1 0 22 1 0 Nervous system disorders Headache 33 2 0 22 1 0 Gastrointestinal disorders Nausea 49 <1 0 47 2 0 Vomiting 25 2 0 23 2 0 Diarrhea 22 1 0 26 6 0 Skin and subcutaneous tissue disorders Alopecia¶ 25 0 0 28 0 0 General disorders and administration site conditions Fatigue# 62 3 0 50 5 0 The following adverse reactions have been identified in <20% of the 286 patients receiving TALZENNA, and thus were not included in Table 3: abdominal pain (19%), dizziness (17%), leukopenia (17%), dysgeusia (10%), dyspepsia (10%), stomatitis (8%), and lymphopenia (7%).
Table 4 Laboratory Abnormalities Reported in ≥25% of Patients in EMBRACA EMBRACA Study TALZENNA
N*=286 (%)Chemotherapy
N*=126 (%)Parameter Grades 1–4 Grade 3 Grade 4 Grades 1–4 Grade 3 Grade 4 Abbreviation: N=number of patients. - * This number represents the safety population. The derived values in the table are based on the total number of evaluable patients for each laboratory parameter.
- † This number represents non-fasting glucose.
Decrease in hemoglobin 90 39 0 77 6 0 Decrease in leukocytes 84 14 0.3 73 22 2 Decrease in neutrophils 68 17 3 70 21 17 Decrease in lymphocytes 76 17 0.7 53 8 0.8 Decrease in platelets 55 11 4 29 2 0 Increase in glucose† 54 2 0 51 2 0 Increase in aspartate aminotransferase 37 2 0 48 3 0 Increase in alkaline phosphatase 36 2 0 34 2 0 Increase in alanine aminotransferase 33 1 0 37 2 0 Decrease in calcium 28 1 0 16 0 0 -
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on TALZENNA
Effect of P-gp Inhibitors
Coadministration with P-gp inhibitors may increase talazoparib exposure.
In patients with advanced solid tumors, coadministration of a P-gp inhibitor (itraconazole) increased talazoparib plasma exposure by 56%. In the clinical studies, coadministration with P-gp inhibitors including amiodarone, carvedilol, clarithromycin, itraconazole, and verapamil resulted in an approximate 45% increase in talazoparib exposure and an increase in the rate of TALZENNA dose reduction. If coadministration of TALZENNA with these P-gp inhibitors cannot be avoided, reduce the TALZENNA dose [see Dosage and Administration (2.5)]. When the P-gp inhibitor is discontinued, increase the TALZENNA dose (after 3–5 half-lives of the inhibitor) to the dose used prior to the initiation of the P-gp inhibitor [see Dosage and Administration (2.5), Clinical Pharmacology (12.3)].
When coadministering TALZENNA with P-gp inhibitors not listed above, monitor patients for potential increased adverse reactions [see Dosage and Administration (2.5), Clinical Pharmacology (12.3)].
Effect of BCRP inhibitors
Coadministration with BCRP inhibitors may increase talazoparib exposure. If coadministration cannot be avoided, monitor patients for potential increased adverse reactions when coadministering [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], TALZENNA can cause embryo-fetal harm when administered to a pregnant woman. There are no available data on TALZENNA use in pregnant women to inform a drug-associated risk. In an animal reproduction study, the administration of talazoparib to pregnant rats during the period of organogenesis caused fetal malformations and structural skeletal variations and embryo-fetal death at maternal exposures that were 0.24 times the AUC in patients receiving the recommended dose of 1 mg daily (see Data). Apprise pregnant women and females of reproductive potential of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the general U.S. population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In an embryo-fetal development toxicity study, pregnant rats received oral doses of 0.015, 0.05, and 0.15 mg/kg/day talazoparib during the period of organogenesis. Talazoparib caused embryo-fetal death at doses ≥0.015 mg/kg/day (approximately 0.24 times the AUC in patients at the recommended dose). A dose of 0.015 mg/kg/day caused decreased fetal body weights and an increased incidence of fetal malformations (depressed eye bulge, small eye, split sternebra, and fused cervical vertebral arch) and structural variations including misshapen or incomplete ossification of the sternebra, skull, rib, and vertebra.
8.2 Lactation
Risk Summary
There are no data on the presence of talazoparib in human milk, the effects of the drug on milk production, or the effects of the drug on the breastfed child. Because of the potential for serious adverse reactions in a breastfed child from talazoparib, advise lactating women not to breastfeed during treatment with TALZENNA and for at least 1 month after the final dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
A pregnancy test is recommended for females of reproductive potential prior to initiating TALZENNA treatment.
Contraception
Females
TALZENNA can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment and for at least 7 months following the last dose of TALZENNA.
Males
Based on genotoxicity and animal reproduction studies, advise male patients with female partners of reproductive potential and pregnant partners to use effective contraception during treatment with TALZENNA and for at least 4 months following the last dose [see Use in Specific Populations (8.1), Nonclinical Toxicology (13.1)].
Infertility
Males
Based on animal studies, TALZENNA may impair fertility in males of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of TALZENNA have not been established in pediatric patients.
8.5 Geriatric Use
In clinical trials of TALZENNA enrolling 494 patients with advanced solid tumors who received TALZENNA 1 mg daily as monotherapy, 85 (17%) patients were ≥65 years of age, and this included 19 (4%) patients who were ≥75 years old. There were 5 patients ≥85 years old. No overall differences in safety or effectiveness of TALZENNA were observed between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
Patients with moderate or severe renal impairment have a higher exposure to TALZENNA than patients with normal renal function. Reduce the recommended dose of TALZENNA in patients with moderate (CLcr 30 – 59 mL/min) and severe (CLcr 15 – 29 mL/min) renal impairment. Monitor patients with severe renal impairment for potential increased adverse reactions and adjust dosing accordingly [see Dosage and Administration (2.4), Clinical Pharmacology (12.3)]. No dose adjustment is required for patients with mild renal impairment (CLcr 60 – 89 mL/min). TALZENNA has not been studied in patients requiring hemodialysis [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
TALZENNA has not been studied in patients with moderate hepatic impairment (total bilirubin >1.5 to 3.0 × upper limit of normal [ULN] and any aspartate aminotransferase [AST]) or severe hepatic impairment (total bilirubin >3.0 × ULN and any AST). No dose adjustment is required for patients with mild hepatic impairment (total bilirubin ≤1 × ULN and AST > ULN, or total bilirubin >1.0 to 1.5 × ULN and any AST) [see Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Talazoparib is an inhibitor of mammalian polyadenosine 5'-diphosphoribose polymerase (PARP) enzyme. The chemical name of talazoparib tosylate is (8S,9R)-5-Fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-2,7,8,9-tetrahydro-3H-pyrido[4,3,2-de]phthalazin-3-one 4-methylbenzenesulfonate (1:1). The chemical formula of talazoparib tosylate is C26H22F2N6O4S, and the relative molecular mass is 552.56 Daltons. The chemical structure of talazoparib tosylate is shown below:
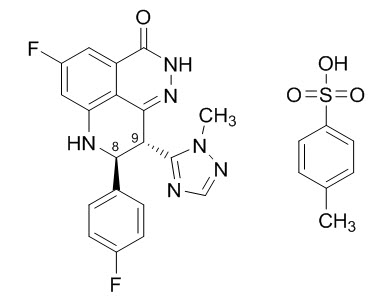
Talazoparib tosylate is a white to yellow solid. TALZENNA capsules for oral use are available as a 0.25 mg hard hypromellose (HPMC) capsule that contains 0.363 mg talazoparib tosylate equivalent to 0.25 mg talazoparib free base or as a 1 mg HPMC capsule that contains 1.453 mg talazoparib tosylate equivalent to 1 mg talazoparib free base.
Inactive ingredients: silicified microcrystalline cellulose (sMCC). The white/ivory and white/light red opaque capsule shells contain HPMC, yellow iron oxide, red iron oxide and titanium dioxide; and the printing ink contains shellac, black iron oxide, potassium hydroxide, ammonium hydroxide, and propylene glycol.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Talazoparib is an inhibitor of poly (ADP-ribose) polymerase (PARP) enzymes, including PARP1 and PARP2, which play a role in DNA repair. In vitro studies with cancer cell lines that harbored defects in DNA repair genes, including BRCA 1 and 2, have shown that talazoparib-induced cytotoxicity may involve inhibition of PARP enzymatic activity and increased formation of PARP-DNA complexes resulting in DNA damage, decreased cell proliferation, and apoptosis. Talazoparib anti-tumor activity was observed in human patient-derived xenograft breast cancer tumor models that expressed mutated or wild-type BRCA 1 and 2.
12.3 Pharmacokinetics
After oral administration of 1 mg TALZENNA once daily in patients, the recommended dose, the geometric mean [% coefficient of variation (CV%)] of AUC and maximum observed plasma concentration (Cmax) of talazoparib at steady-state was 208 (37%) ng.hr/mL and 16.4 (32%) ng/mL, respectively. The pharmacokinetics (PK) of talazoparib is linear from 0.025 mg to 2 mg (2 times the recommended dose). The median accumulation ratio of talazoparib following repeated oral administration of 1 mg once daily was in the range of 2.3 to 5.2. Talazoparib plasma concentrations reached steady-state within 2 to 3 weeks.
Absorption
Following oral administration of talazoparib, the median time to Cmax (Tmax) was generally between 1 to 2 hours after dosing.
Food Effect
Following a single oral dose of 0.5 mg TALZENNA with high-fat, high-calorie food (approximately 800 to 1000 calories with 150, 250, and 500 to 600 calories from protein, carbohydrate, and fat, respectively), the mean Cmax of talazoparib was decreased by 46%, the median Tmax was delayed from 1 to 4 hours, and AUCinf was not affected.
Distribution
The mean apparent volume of distribution of talazoparib is 420 L. In vitro, protein binding of talazoparib is 74% and is independent of talazoparib concentration.
Elimination
The mean terminal plasma half-life (±standard deviation) of talazoparib is 90 (±58) hours, and the mean apparent oral clearance (inter-subject variability) is 6.45 L/h (31.1%) in cancer patients.
Specific Populations
Age (18 to 88 years), sex, race (361 White, 41 Asian, 16 Black, 9 Others, and 63 Not Reported), and body weight (36 to 162 kg) had no clinically relevant effect on the PK of talazoparib.
Patients with Renal Impairment
Talazoparib steady-state total exposure (AUC0–24) increased by 12%, 43%, and 163% in patients with mild (eGFR 60 – 89 mL/min/1.73 m2), moderate (eGFR 30 – 59 mL/min/1.73 m2), and severe (eGFR 15 – 29 mL/min/1.73 m2) renal impairment, respectively, relative to patients with normal renal function (eGFR ≥ 90 mL/min/1.73 m2). Talazoparib steady-state peak concentration (Cmax) increased by 11%, 32%, and 89% in patients with mild, moderate, and severe renal impairment, respectively, relative to patients with normal renal function. The PK of talazoparib has not been studied in patients requiring hemodialysis. There was no evidence of a relationship between the protein binding of talazoparib and renal function.
Patients with Hepatic Impairment
Mild hepatic impairment (total bilirubin ≤1.0 × ULN and AST > ULN, or total bilirubin >1.0 to 1.5 × ULN and any AST) had no effect on the PK of talazoparib. The PK of talazoparib have not been studied in patients with moderate (total bilirubin >1.5 to 3.0 × ULN and any AST) or severe hepatic impairment (total bilirubin >3.0 × ULN and any AST).
Drug Interaction Studies
Effect of Other Drugs on Talazoparib
Effect of P-gp inhibitors: In patients with advanced solid tumors, coadministration of a P-gp inhibitor (multiple 100 mg twice-daily doses of itraconazole) with a single 0.5 mg talazoparib dose increased talazoparib AUCinf and Cmax by approximately 56% and 40%, respectively. Population PK analysis showed that coadministration with P-gp inhibitors including amiodarone, carvedilol, clarithromycin, itraconazole, and verapamil in clinical studies increased talazoparib exposure by 45% [see Dosage and Administration (2.5), Drug Interactions (7.1)].
Coadministration with P-gp inhibitors including azithromycin, atorvastatin, diltiazem, felodipine, fluvoxamine, and quercetin in clinical studies increased talazoparib exposure by 8% [see Dosage and Administration (2.5), Drug Interactions (7)].
Effect of P-gp inducers: In patients with advanced solid tumors, coadministration of a P-gp inducer (multiple 600 mg once-daily doses of rifampin) with a single 1 mg talazoparib dose increased talazoparib Cmax by 37% with no effect on talazoparib exposure.
Effect of BCRP inhibitors: The effect of BCRP inhibitors on PK of talazoparib has not been studied. Coadministration with BCRP inhibitors may increase talazoparib exposure [see Drug Interactions (7)].
Effect of acid-reducing agents on talazoparib: Coadministration of acid-reducing agents including proton pump inhibitors (PPI), histamine receptor 2 antagonists (H2RA), or other acid reducing agents has no effect on the absorption of talazoparib.
In Vitro Studies
Talazoparib is a substrate of P-gp and BCRP transporters.
Talazoparib is not a substrate of organic anion transporting polypeptide [OATP]1B1, OATP1B3, organic cationic transporter [OCT]1, OCT2, organic anion transporter [OAT]1, OAT3, bile salt export pump [BSEP], multidrug and toxin extrusion [MATE]1, and MATE2-K.
Talazoparib is not an inhibitor of cytochrome (CYP)1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A4/5, or inducer of CYP1A2, CYP2B6, or CYP3A4.
Talazoparib is not an inhibitor of transporters including P-gp, BCRP, OATP1B1, OATP1B3, OCT1, OCT2, OAT1, OAT3, BSEP, MATE1, and MATE2-K.
Talazoparib is not an inhibitor of uridine-diphosphate glucuronosyltransferase (UGT) isoforms (1A1, 1A4, 1A6, 1A9, 2B7, and 2B15).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with talazoparib.
Talazoparib was clastogenic in an in vitro chromosomal aberration assay in human peripheral blood lymphocytes and in an in vivo bone marrow micronucleus assay in rats. This clastogenicity is consistent with genomic instability resulting from the primary pharmacology of talazoparib, indicating the potential for genotoxicity in humans. Talazoparib was not mutagenic in a bacterial reverse mutation (Ames) test.
Fertility studies in animals have not been conducted with talazoparib. In repeat-dose toxicity studies up to 3-months duration, talazoparib-related findings in the testis and epididymis at doses ≥0.04 mg/kg/day in rats and ≥0.01 mg/kg/day in dogs included decreased organ weights, luminal cellular debris, reduced sperm, and degeneration/atrophy. These doses in rats and dogs resulted in approximately 1.0 times and 0.2 times, respectively, the exposure (AUC) in humans at the recommended dose. Follicular atresia of the ovary was observed in rats at doses ≥1 mg/kg/day talazoparib, approximately 9.5 times the AUC in patients at the recommended dose.
-
14 CLINICAL STUDIES
EMBRACA Study (NCT01945775)
Deleterious or Suspected Deleterious Germline BRCA-mutated (gBRCAm) HER2-negative Locally Advanced or Metastatic Breast Cancer
EMBRACA (NCT01945775) was an open-label study in which patients (N=431) with gBRCAm HER2-negative locally advanced or metastatic breast cancer were randomized 2:1 to receive TALZENNA 1 mg or healthcare provider's choice of chemotherapy (capecitabine, eribulin, gemcitabine, or vinorelbine) until disease progression or unacceptable toxicity. Randomization was stratified by prior use of chemotherapy for metastatic disease (0 versus 1, 2, or 3), by triple-negative disease status (triple-negative breast cancer [TNBC] versus non-TNBC), and history of central nervous system (CNS) metastasis (yes versus no).
Patients received no more than 3 prior cytotoxic chemotherapy regimens for their metastatic or locally advanced disease. Patients were required to have received treatment with an anthracycline and/or a taxane (unless contraindicated) in the neoadjuvant, adjuvant, and/or metastatic treatment setting. First-line treatment for advanced or metastatic disease with no prior adjuvant chemotherapy was allowed if the investigator determined that 1 of the 4 chemotherapy choices in the control arm would be an appropriate treatment option for the patient. Patients with prior platinum therapy for advanced disease were required to have no evidence of disease progression during platinum therapy. No prior treatment with a PARP inhibitor was permitted. Of the 431 patients randomized in the EMBRACA study, 408 (95%) were centrally confirmed to have a deleterious or suspected deleterious gBRCAm using a clinical trial assay; out of which 354 (82%) were confirmed using the BRACAnalysis CDx®. BRCA mutation status (breast cancer susceptibility gene 1 [BRCA1] positive or breast cancer susceptibility gene 2 [BRCA2] positive) was similar across both treatment arms.
The median age of patients treated with TALZENNA was 45 years (range 27 to 84) and 50 years (range 24 to 88) among patients treated with chemotherapy. Among all randomized patients, 1% versus 2% were males, 67% versus 75% were White; 11% versus 11% were Asian, and 4% versus 1% were Black or African American in the TALZENNA and chemotherapy arms, respectively. Almost all patients (98%) in both arms had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Approximately 56% of patients had estrogen receptor-positive and/or progesterone receptor-positive disease; 44% of patients had triple-negative disease, and the proportions were balanced across both treatment arms. Fifteen percent (15%) of patients in the TALZENNA arm and 14% of patients in the chemotherapy arm had a history of CNS metastases. Ninety-one percent (91%) of patients in the TALZENNA arm had received prior taxane therapy, and 85% had received prior anthracycline therapy in any setting. Sixteen percent (16%) of patients in the TALZENNA arm and 21% of patients in the chemotherapy arm had received prior platinum treatment in any setting. The median number of prior cytotoxic regimens for patients with advanced breast cancer was one; 38% received no prior cytotoxic regimens for advanced or metastatic disease, 37% received one, 20% received two, and 5% received three or more prior cytotoxic regimens.
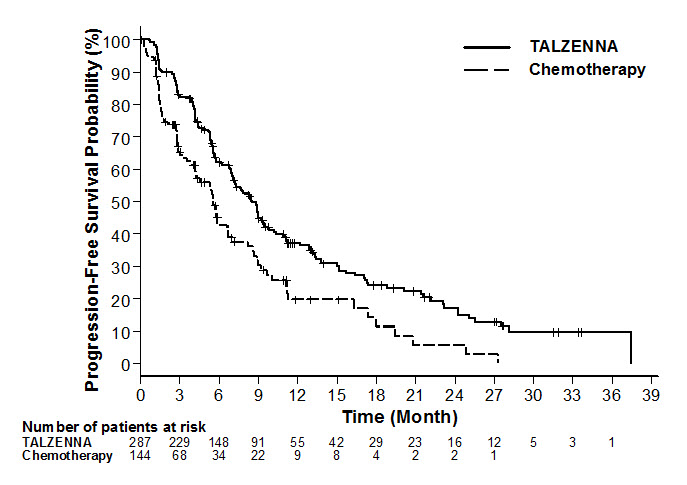
The major efficacy outcome measure was progression-free survival (PFS) evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, as assessed by blinded independent central review (BICR). A statistically significant improvement in PFS was demonstrated for TALZENNA compared with chemotherapy. A sensitivity analysis of investigator-assessed PFS was consistent with the BICR-assessed PFS results. Consistent PFS results were observed across patient subgroups defined by study stratification factors (line of therapy, TNBC status, and history of CNS metastases). The overall survival (OS) data were not mature at the time of the final PFS analysis (38% of patients had died). Efficacy data from the EMBRACA study are summarized in Table 5, and the Kaplan-Meier curves for PFS are shown in Figure 1.
Table 5. Summary of Efficacy Results – EMBRACA Study TALZENNA Chemotherapy Abbreviations: BICR=blinded independent central review; CI=confidence interval. - * Hazard ratio is estimated from a Cox proportional hazards model stratified by prior use of chemotherapy for metastatic disease (0 versus 1, 2, or 3), by triple-negative disease status (triple-negative breast cancer [TNBC] versus non TNBC), and by history of central nervous system metastasis (yes versus no).
- † P-values from stratified log-rank test (2-sided).
- ‡ Conducted in intent-to-treat (ITT) population with measurable disease at baseline.
- § Response rate based on confirmed responses.
- ¶ Median estimated from Kaplan-Meier probabilities.
Progression-Free Survival by BICR N=287 N=144 Events, number (%) 186 (65) 83 (58) Median months (95% CI) 8.6 (7.2, 9.3) 5.6 (4.2, 6.7) Hazard Ratio (95% CI)* 0.54 (0.41, 0.71) p-value† p<0.0001 Patients with Measurable Disease by Investigator‡ N=219 N=114 Objective Response Rate, % (95% CI)§ 50.2 (43.4, 57.0) 18.4 (11.8, 26.8) Duration of Response Median¶ months (95% CI) 6.4 (5.4, 9.5) 3.9 (3.0, 7.6) Figure 1. Kaplan-Meier Curves of PFS – EMBRACA Study
-
16 HOW SUPPLIED/STORAGE AND HANDLING
TALZENNA is supplied in strengths and package configurations as described in Table 6:
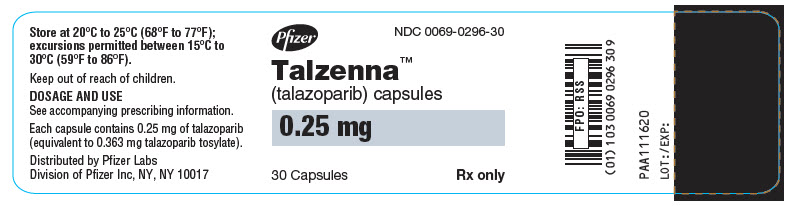
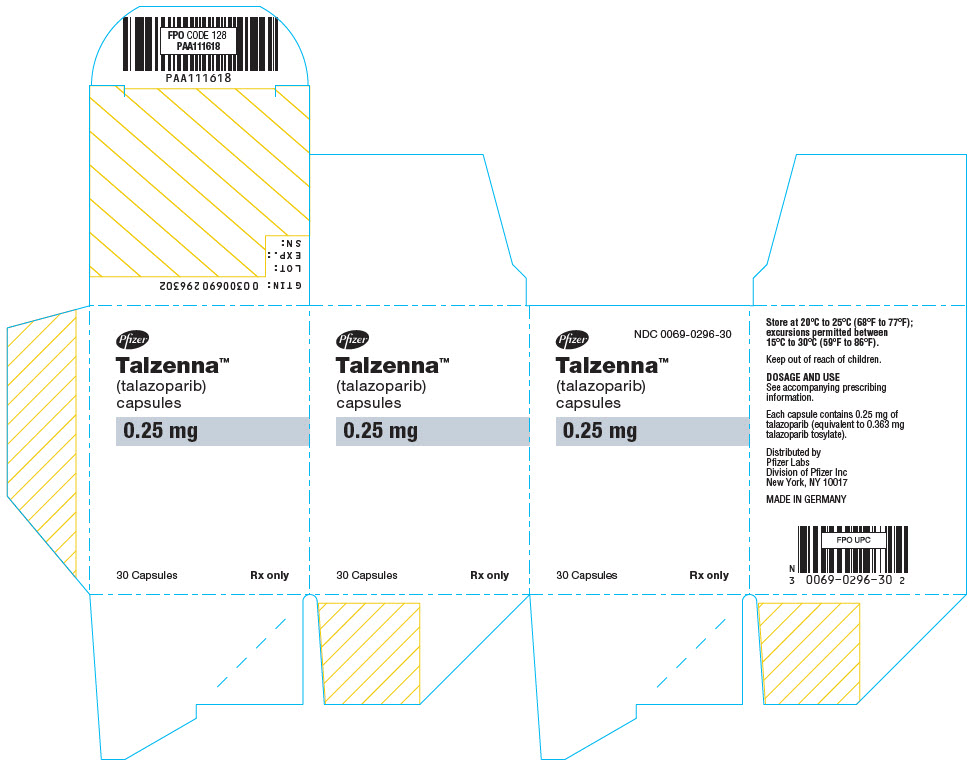
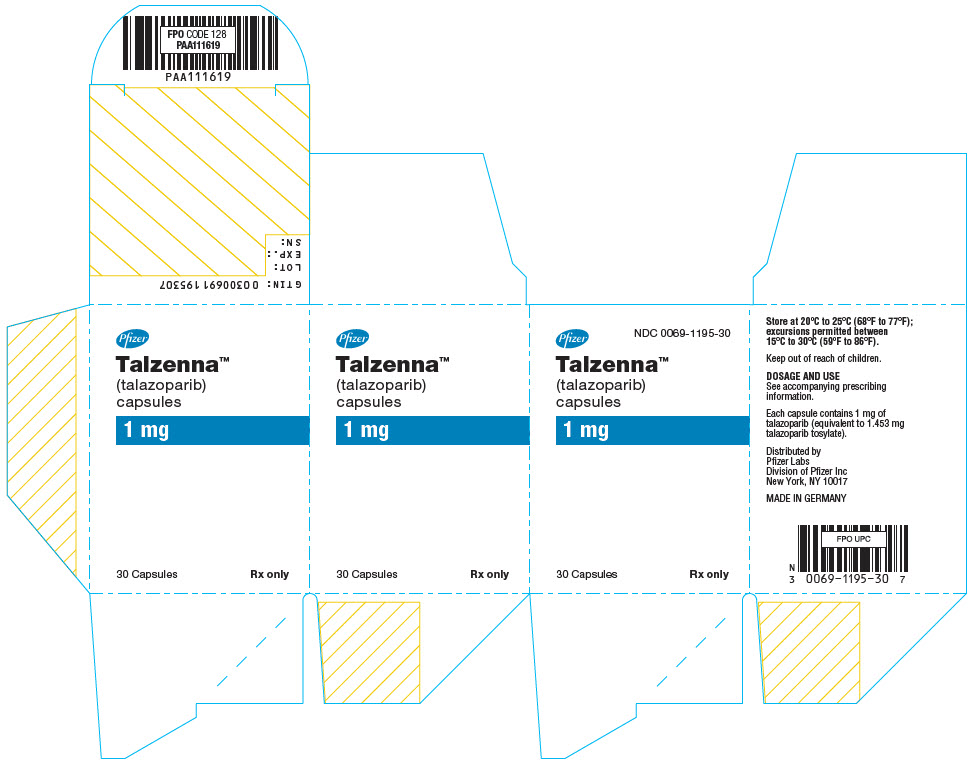
Table 6. TALZENNA Capsules Package Configuration Capsule Strength (mg) NDC Print Bottles of 30 capsules 0.25 NDC: 0069-0296-30 Ivory cap (printed with "Pfizer" in black) and a white body (printed with "TLZ 0.25" in black). Bottles of 30 capsules 1 NDC: 0069-1195-30 Light red cap (printed with "Pfizer" in black) and a white body (printed with "TLZ 1" in black). -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- MDS/AML: Advise patients to contact their healthcare provider if they experience weakness, feeling tired, fever, weight loss, frequent infections, bruising, bleeding easily, breathlessness, blood in urine or stool, and/or laboratory findings of low blood cell counts, or a need for blood transfusions. This may be a sign of hematological toxicity or a more serious uncommon bone marrow problem called MDS or AML, which have been reported in patients who received PARP inhibitors [see Warnings and Precautions (5.1)].
- Myelosuppression: Advise patients that TALZENNA may affect hematopoiesis and can cause anemia, leukopenia/neutropenia, and/or thrombocytopenia [see Warnings and Precautions (5.2)].
- Administration Instructions: Advise patients that TALZENNA can be taken once daily with or without food. Instruct patients that if they miss a dose of TALZENNA, they should take their next normal dose at the usual time. Also advise patients to swallow each capsule whole, and that capsules must not be opened or dissolved [see Dosage and Administration (2.2)].
- Embryo-Fetal Toxicity: Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the risk to a fetus and potential loss of the pregnancy [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with TALZENNA and for at least 7 months after the last dose. Advise male patients with female partners of reproductive potential or who are pregnant to use effective contraception during treatment and for at least 4 months after receiving the last dose of TALZENNA [see Warnings and Precautions (5.3), Use in Specific Populations (8.1, 8.3)].
- Lactation: Advise patients not to breastfeed while taking TALZENNA and for at least 1 month after receiving the last dose [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Issued 3/2020 PATIENT INFORMATION
TALZENNA® (Tal-ZEN-ah)
(talazoparib)
capsulesWhat is the most important information I should know about TALZENNA?
TALZENNA may cause serious side effects, including:
Bone marrow problems called Myelodysplastic Syndrome (MDS) or Acute Myeloid Leukemia (AML). Some people who have cancer and who have received previous treatment with chemotherapy or certain other medicines for their cancer have developed MDS or AML during or after treatment with TALZENNA. MDS or AML may lead to death. If you develop MDS or AML, your healthcare provider will stop treatment with TALZENNA.
Symptoms of low blood cell counts are common during treatment with TALZENNA, but can be a sign of serious problems, including MDS or AML. Tell your healthcare provider if you have any of the following symptoms during treatment with TALZENNA:- weakness
- weight loss
- fever
- frequent infections
- blood in urine or stool
- shortness of breath
- feeling very tired
- bruising or bleeding more easily
Your healthcare provider will do blood tests to check your blood cell counts: - before treatment with TALZENNA
- every month during treatment with TALZENNA
- weekly if you have low blood cell counts that last a long time. Your healthcare provider may stop treatment with TALZENNA until your blood cell counts improve.
What is TALZENNA?
TALZENNA is a prescription medicine used to treat adults with:- a certain type of breast cancer (human epidermal growth factor receptor 2 [HER2]-negative), and
- an abnormal inherited BRCA gene, and
- whose cancer has spread to other parts of the body (locally advanced or metastatic).
It is not known if TALZENNA is safe and effective in children.Before taking TALZENNA, tell your healthcare provider about all of your medical conditions, including if you: - have kidney problems
- are pregnant or plan to become pregnant. TALZENNA can harm your unborn baby, and may cause loss of pregnancy (miscarriage). You should not become pregnant during treatment with TALZENNA. Tell your healthcare provider right away if you are pregnant or become pregnant during treatment with TALZENNA.
- If you are able to become pregnant, your healthcare provider may do a pregnancy test before you start treatment with TALZENNA.
- Females who are able to become pregnant should use effective birth control (contraception) during treatment with TALZENNA and for at least 7 months after receiving the last dose of TALZENNA. Talk to your healthcare provider about forms of birth control that may be right for you.
- Males with female partners who are pregnant or are able to become pregnant should use effective birth control during treatment with TALZENNA and for at least 4 months after receiving the last dose of TALZENNA.
- are breastfeeding or plan to breastfeed. It is not known if TALZENNA passes into your breast milk. Do not breastfeed during treatment with TALZENNA and for at least 1 month after receiving the last dose of TALZENNA. Talk to your healthcare provider about the best way to feed your baby during this time.
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.How should I take TALZENNA? - Take TALZENNA exactly as your healthcare provider tells you.
- Do not change your dose or stop taking TALZENNA without first talking with your healthcare provider.
- Take TALZENNA 1 time a day.
- Take TALZENNA with or without food.
- Swallow TALZENNA capsules whole. Do not dissolve or open TALZENNA capsules.
- Your healthcare provider may change your dose of TALZENNA or tell you to stop taking TALZENNA depending on how you respond to treatment.
- If you miss a dose of TALZENNA or vomit, take your next dose at your regular time. Do not take an extra dose to make up for a missed dose.
- If you take too much TALZENNA, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of TALZENNA?
TALZENNA may cause serious side effects, including:
See "What is the most important information I should know about TALZENNA?"
The most common side effects of TALZENNA include:- tiredness or weakness
- low number of red or white blood cells
- nausea
- low number of platelets
- headache
- loss of appetite
- diarrhea
- vomiting
- hair loss
TALZENNA may cause fertility problems in males. This may affect your ability to father a child. Talk to your healthcare provider if this is a concern for you. These are not all of the possible side effects of TALZENNA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store TALZENNA?
Store TALZENNA at 68°F to 77°F (20°C to 25°C ).
Keep TALZENNA and all medicines out of the reach of children.General information about the safe and effective use of TALZENNA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use TALZENNA for a condition for which it is not prescribed. Do not give TALZENNA to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about TALZENNA that is written for health professionals.What are the ingredients in TALZENNA?
Active ingredient: talazoparib tosylate
Inactive ingredients: silicified microcrystalline cellulose (sMCC). The white and ivory and white and light red opaque capsule shells contain hypromellose (HPMC), yellow iron oxide, red iron oxide and titanium dioxide. The printing ink contains shellac, black iron oxide, potassium hydroxide, ammonium hydroxide, and propylene glycol.

For more information, go to www.TALZENNA.com or call 1-800-438-1985.
LAB-1272-2.0 - PRINCIPAL DISPLAY PANEL - 0.25 mg Capsule Bottle Label
- PRINCIPAL DISPLAY PANEL - 0.25 mg Capsule Bottle Carton
- PRINCIPAL DISPLAY PANEL - 1 mg Capsule Bottle Label
- PRINCIPAL DISPLAY PANEL - 1 mg Capsule Bottle Carton
-
INGREDIENTS AND APPEARANCE
TALZENNA
talazoparib capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0069-0296 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TALAZOPARIB TOSYLATE (UNII: 02WK9U5NZC) (TALAZOPARIB - UNII:9QHX048FRV) TALAZOPARIB 0.25 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) AMMONIA (UNII: 5138Q19F1X) PROPYLENE GLYCOL, (R)- (UNII: 602HN5L69H) Product Characteristics Color WHITE (ivory cap) , WHITE (white body) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code Pfizer;TLZ;025 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0069-0296-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 10/26/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211651 10/26/2018 TALZENNA
talazoparib capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0069-1195 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TALAZOPARIB TOSYLATE (UNII: 02WK9U5NZC) (TALAZOPARIB - UNII:9QHX048FRV) TALAZOPARIB 1 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) AMMONIA (UNII: 5138Q19F1X) PROPYLENE GLYCOL, (R)- (UNII: 602HN5L69H) Product Characteristics Color RED (light red cap) , WHITE (white body) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code Pfizer;TLZ;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0069-1195-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 10/26/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211651 10/26/2018 Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) Establishment Name Address ID/FEI Business Operations Pfizer Pharmaceuticals LLC 829084552 LABEL(0069-0296, 0069-1195) , PACK(0069-0296, 0069-1195) Establishment Name Address ID/FEI Business Operations Pharmacia and Upjohn Company LLC 618054084 LABEL(0069-0296, 0069-1195) , PACK(0069-0296, 0069-1195)
Trademark Results [Talzenna]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TALZENNA 87293680 not registered Dead/Abandoned |
Wyeth LLC 2017-01-09 |
 TALZENNA 86253764 5291322 Live/Registered |
Wyeth LLC 2014-04-16 |
 TALZENNA 85173532 not registered Dead/Abandoned |
Wyeth LLC 2010-11-10 |
 TALZENNA 77180181 not registered Dead/Abandoned |
WYETH LLC 2007-05-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.