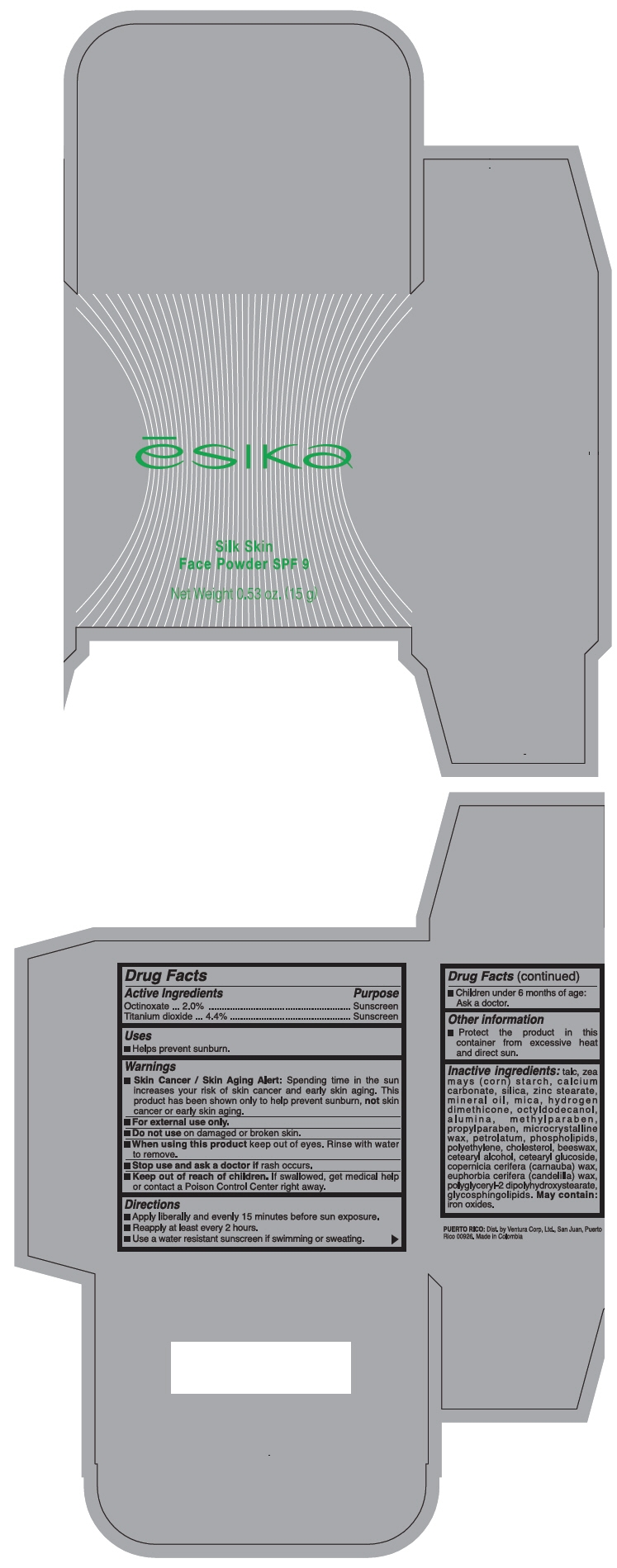
ESIKA SILK SKIN FACE SPF 9- octinoxate and titanium dioxide powder
ESIKA Silk Skin Face SPF 9 by
Drug Labeling and Warnings
ESIKA Silk Skin Face SPF 9 by is a Otc medication manufactured, distributed, or labeled by Ventura Corporation LTD, Bel Star S.A. (Colombia). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
TALC, ZEA MAYS (CORN) STARCH, CALCIUM CARBONATE, SILICA, ZINC STEARATE, MINERAL OIL, MICA, HYDROGEN DIMETHICONE, OCTYLDODECANOL, ALUMINA, METHYLPARABEN, PROPYLPARABEN, MICROCRYSTALLINE WAX, PETROLATUM, PHOSPHOLIPIDS, POLYETHYLENE, CHOLESTEROL, BEESWAX, CETEARYL ALCOHOL, CETEARYL GLUCOSIDE, COPERNICIA CERIFERA (CARNAUBA) WAX, EUPHORBIA CERIFERA (CANDELILLA) WAX, POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE, GLYCOSPHINGOLIPIDS
MAY CONTAIN: IRON OXIDES
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 15 g Case Box
-
INGREDIENTS AND APPEARANCE
ESIKA SILK SKIN FACE SPF 9
octinoxate and titanium dioxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-176 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.02 g in 1 g Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 0.044 g in 1 g Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) STARCH, CORN (UNII: O8232NY3SJ) CALCIUM CARBONATE (UNII: H0G9379FGK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ZINC STEARATE (UNII: H92E6QA4FV) MINERAL OIL (UNII: T5L8T28FGP) MICA (UNII: V8A1AW0880) OCTYLDODECANOL (UNII: 461N1O614Y) ALUMINUM OXIDE (UNII: LMI26O6933) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) PETROLATUM (UNII: 4T6H12BN9U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CHOLESTEROL (UNII: 97C5T2UQ7J) YELLOW WAX (UNII: 2ZA36H0S2V) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CARNAUBA WAX (UNII: R12CBM0EIZ) CANDELILLA WAX (UNII: WL0328HX19) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-176-02 1 in 1 BOX 10/31/2016 1 NDC: 13537-176-01 15 g in 1 CASE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 10/31/2016 Labeler - Ventura Corporation LTD (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(13537-176)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.