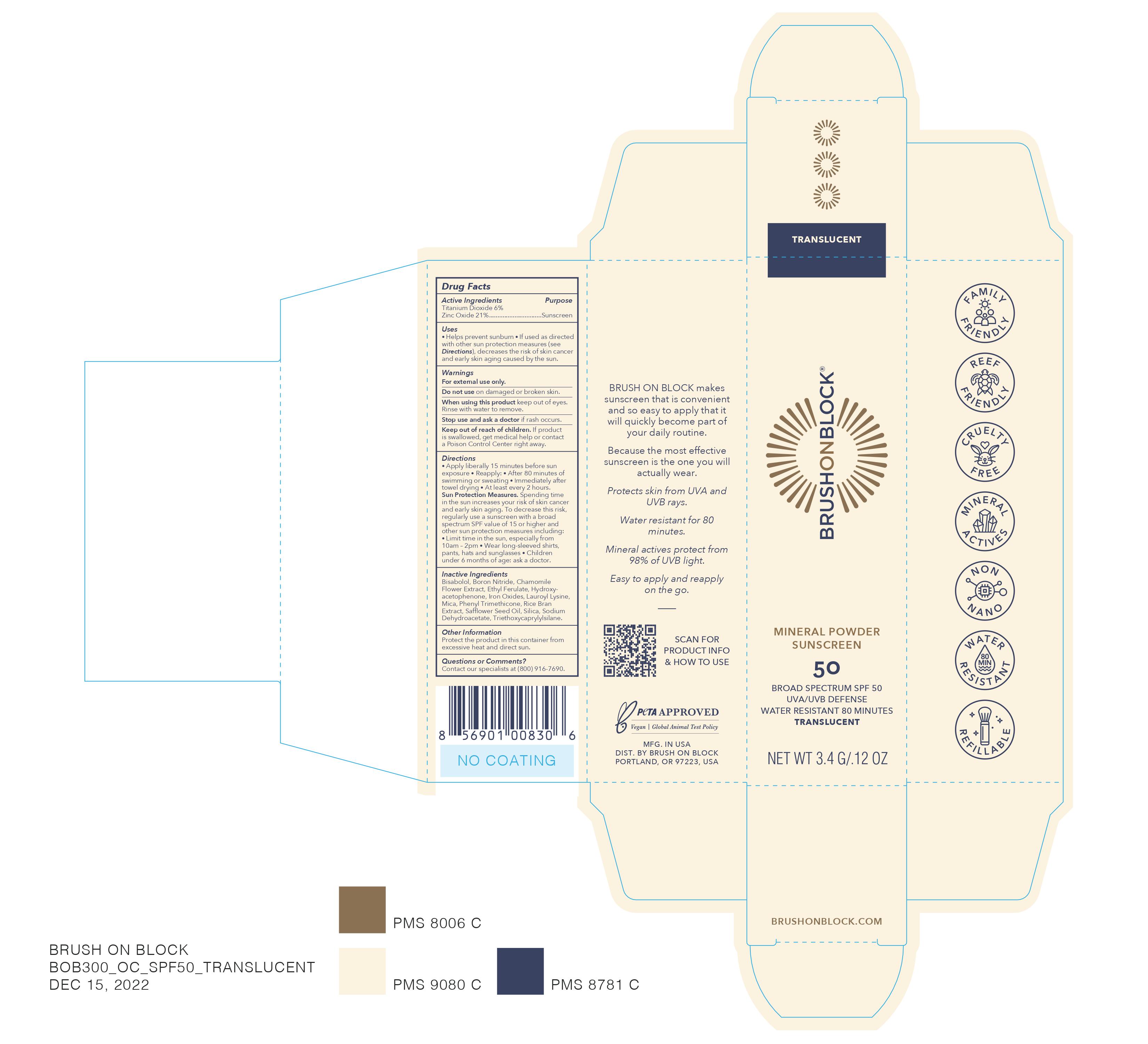
Brush on Block Mineral Powder Sunscreen Translucent
Brush on Block Mineral Powder Sunscreen Translucent by
Drug Labeling and Warnings
Brush on Block Mineral Powder Sunscreen Translucent by is a Otc medication manufactured, distributed, or labeled by SPF Ventures, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BRUSH ON BLOCK MINERAL POWDER SUNSCREEN TRANSLUCENT- titanium dioxide, zinc oxide powder
SPF Ventures, LLC
----------
Brush on Block Mineral Powder Sunscreen Translucent
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early aging caused by the sun.
Directions
- Apply liberally 15 minutes before sun exposure
- Reapply: after 80 minutes of swimming or sweating Immediately after towel drying At least every 2 hours.
- Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including: Limit time in the sun, especially from 10am - 2pm Wear long-sleeved shirts, pants, hats, and sunglasses Children under 6 months: Ask a doctor
Directions
Apply liberally 15 minutes before sun exposure
Reapply: after 80 minutes of swimming or sweating Immediately after towel drying At least every 2 hours.
Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including: Limit time in the sun, especially from 10am - 2pm Wear long-sleeved shirts, pants, hats, and sunglasses Children under 6 months: Ask a doctor
| BRUSH ON BLOCK MINERAL POWDER SUNSCREEN TRANSLUCENT
titanium dioxide, zinc oxide powder |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - SPF Ventures, LLC (055483891) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.