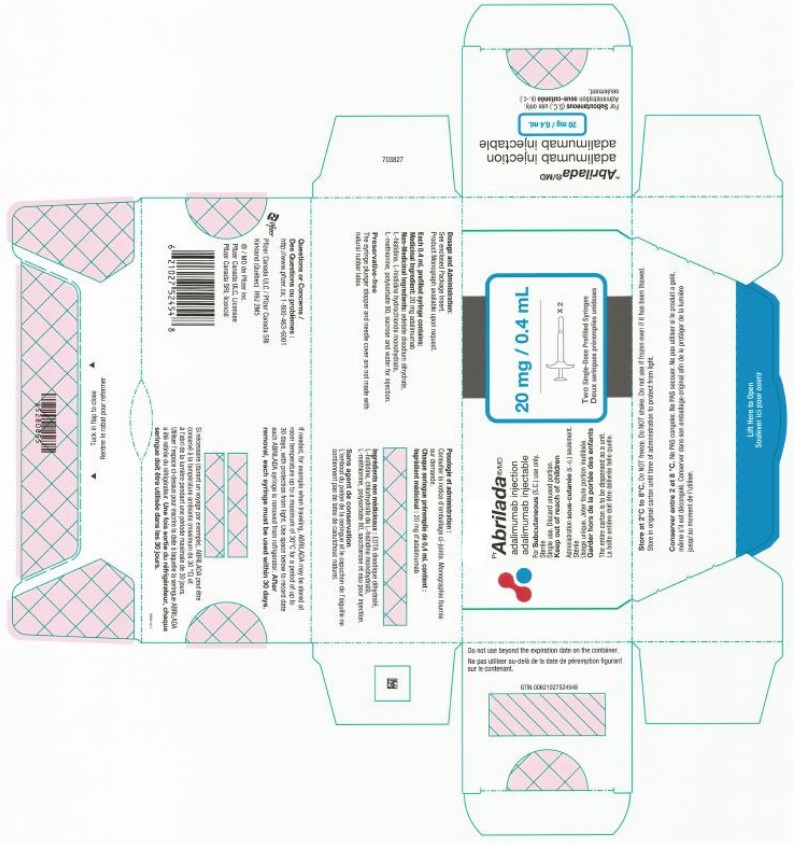
Abrilada by Catalent Indiana, LLC
Abrilada by
Drug Labeling and Warnings
Abrilada by is a Prescription medication manufactured, distributed, or labeled by Catalent Indiana, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ABRILADA- adalimumab-afzb injection, solution
Catalent Indiana, LLC
----------
| ABRILADA
adalimumab-afzb injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Catalent Indiana, LLC (172209277) |
Revised: 9/2023
Document Id: 16c2517e-623a-4c5c-a714-017c71f65d5d
Set id: f2ee682a-8ea6-4068-bdbe-8a9e7d1a2514
Version: 7
Effective Time: 20230926
Trademark Results [Abrilada]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ABRILADA 90229278 not registered Live/Pending |
Pfizer Inc. 2020-10-01 |
 ABRILADA 88907943 not registered Live/Pending |
Pfizer Inc. 2020-05-08 |
 ABRILADA 87482653 not registered Live/Pending |
Pfizer Inc. 2017-06-09 |
 ABRILADA 86578635 not registered Dead/Abandoned |
Pfizer Inc. 2015-03-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.