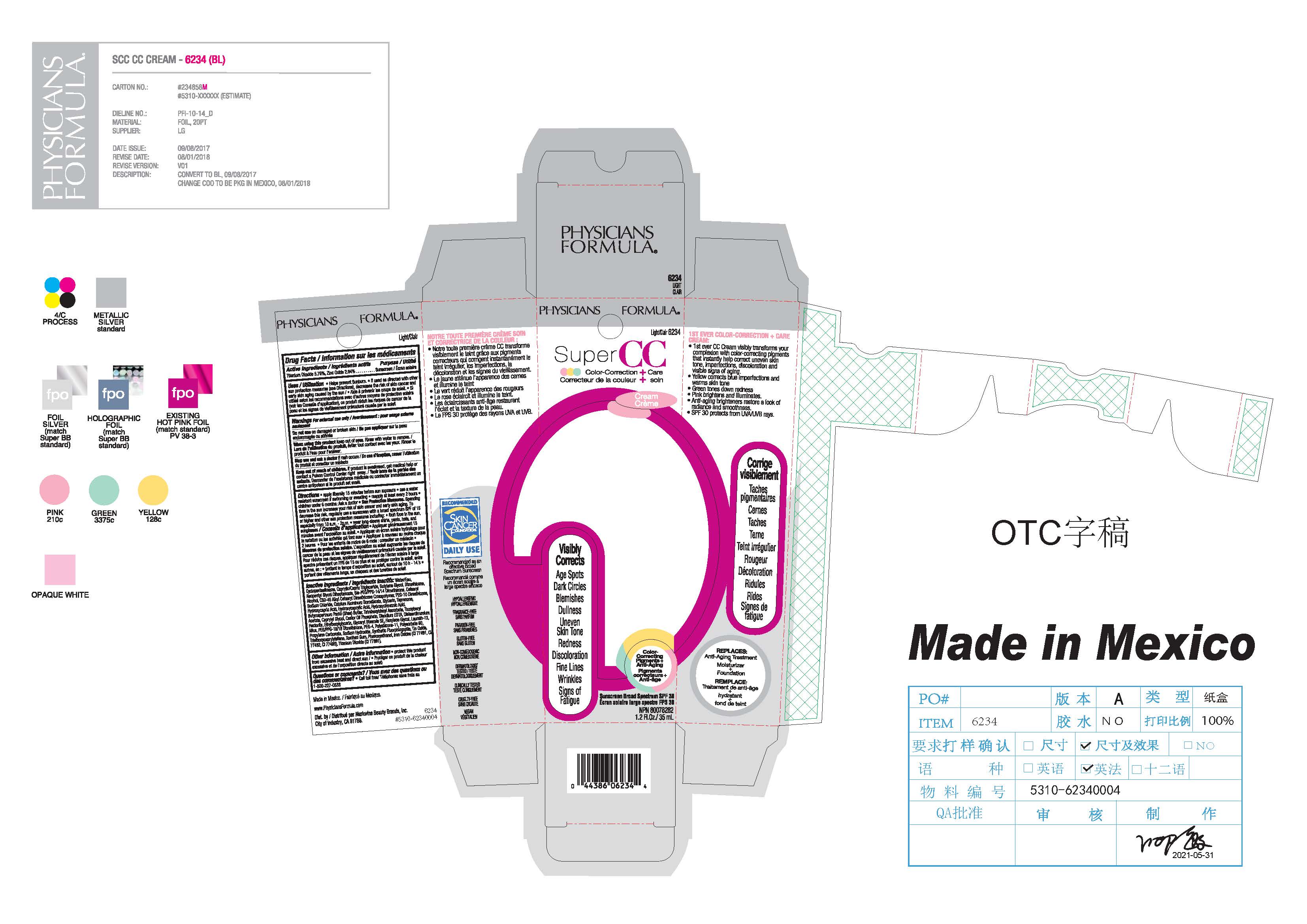
Super CC Cream-6234, 6235, 69514-301
Super CC Color Correction Care Cream by
Drug Labeling and Warnings
Super CC Color Correction Care Cream by is a Otc medication manufactured, distributed, or labeled by Markwins Beauty Brands. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SUPER CC COLOR CORRECTION CARE CREAM- titanium dioxide, zinc oxide cream
Markwins Beauty Brands
----------
Super CC Cream-6234, 6235, 69514-301
apply liberally 15 minutes before sun exposure
use a water resistant sunscreen if swimming or sweating
reapply at least every 2 hours
children under 6 months: Ask a doctor
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broda spectrum SPF of 15 or higher and other sun protection measures including limit time in the sun, especially from 10 a.m. - 2 p.m., wear long-sleeve shirts, pants, hats, and sunglasses
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove
Stop use and ask a doctor if rash occurs
Water/Eau, Cyclopentasiloxane, Caprylic/Capric Triglyceride, Butylene Glycol, Dimethicone, Neopentyl Glycol Diheptanoate, Bis-PEG/PPG-14/14 Dimethicone, Cetearyl Alcohol, C30-45 Alkyl Cetearyl Dimethicone Crosspolymer, PEG-10 Dimethicone, Sodium Chloride, Calcium Aluminum Borosilicate, Glycerin, Teprenone, Hydroxycapric Acid, Hydroxycaprylic Acid, Hydroxycinnamic Acid, Butyrospermum Parkii (Shea) Butter, Tetrahexyldecyl Ascorbate, Tocopheryl Acetate, Caprylyl Glycol, Castor Oil Phosphate, Disodium EDTA, Disteardimonium Hectorite, Ethylhexylglycerin, Glyceryl Stearate SE, Hexylene Glycol, Laureth-12, Mica, PEG/PPG-18/18 Dimethicone, PEG-4, Polysilicone-11, Polysorbate 60, Propylene Carbonate, Sodium Hydroxide, Synthetic Fluorphlogopite, Tin Oxide, Triethoxycaprylylsilane, Xanthan Gum, Phenoxyethanol, Iron Oxides (CI 77491, CI 77492, CI 77499), Titanium Dioxide (CI 77891).
| SUPER CC COLOR CORRECTION CARE CREAM
titanium dioxide, zinc oxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Markwins Beauty Brands (078890119) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.