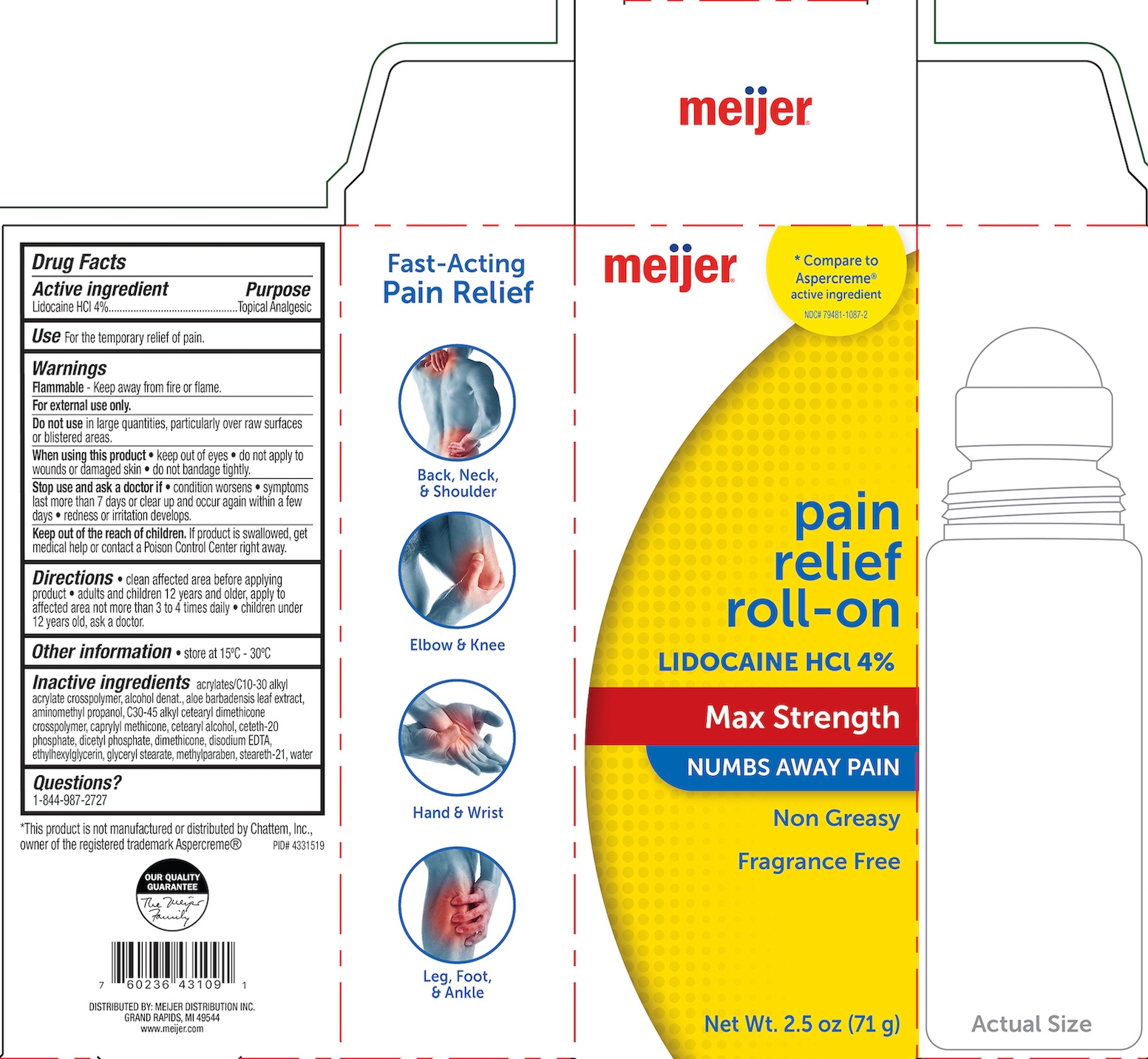
MEIJER PAIN RELIEF- lidocaine hcl 4% gel
Meijer Pain Relief by
Drug Labeling and Warnings
Meijer Pain Relief by is a Otc medication manufactured, distributed, or labeled by Meijer, Derma Care Research Labs, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
For external use only. Flammable--Do not use while smoking or near heat or flame. When using this product avoid contact with eyes, do not apply to wounds or damaged skin, and do not bandage tightly. Stop use and ask a doctor if condition worsens, if symptoms persist for more than 7 days or clear up and occur again within a few days, redness or irritation develops.
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Acrylates/C10-30 alkyl acrylate crosspolymer, alcohol denat., aloe barbadensis leaf extract, aminomethyl propanol, C30-45 alkyl cetearyl dimehticone crosspolymer, carpylyl methicone, cetearyl alcohol, ceteth-20 phosphate, dicetyl phosphate, dimethicone, disodium EDTA, ethylhexylglycerin, glyceryl stearate, methylparaben, steareth-21, water.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MEIJER PAIN RELIEF
lidocaine hcl 4% gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 79481-1087 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 4 g in 100 g Inactive Ingredients Ingredient Name Strength DIMETHICONE 200 (UNII: RGS4T2AS00) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 809Y72KV36) ALCOHOL (UNII: 3K9958V90M) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) C30-45 ALKYL CETEARYL DIMETHICONE CROSSPOLYMER (UNII: 4ZK9VP326R) WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETETH-20 PHOSPHATE (UNII: 921FTA1500) ALOE VERA LEAF (UNII: ZY81Z83H0X) DIHEXADECYL PHOSPHATE (UNII: 2V6E5WN99N) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) METHYLPARABEN (UNII: A2I8C7HI9T) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) STEARETH-21 (UNII: 53J3F32P58) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 79481-1087-2 71 g in 1 BOTTLE; Type 0: Not a Combination Product 08/31/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 08/31/2021 Labeler - Meijer (006959555) Registrant - Derma Care Research Labs, LLC (116817470) Establishment Name Address ID/FEI Business Operations Derma Care Research Labs, LLC 116817470 manufacture(79481-1087)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.