TRIENTINE HYDROCHLORIDE capsule
Trientine Hydrochloride by
Drug Labeling and Warnings
Trientine Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Rising Pharma Holdings, Inc., Suven Life Sciences. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Trientine hydrochloride, USP is N,N'-bis (2-aminoethyl)-1,2-ethanediamine dihydrochloride. It is a white to pale yellow crystalline hygroscopic powder. It is freely soluble in water, soluble in methanol, slightly soluble in ethanol, and insoluble in chloroform and ether.
The molecular formula is C6H18N42HCl with a molecular weight of 219.2. The structural formula is: NH2(CH2)2NH(CH2)2NH(CH2)2NH22HCl
Trientine hydrochloride, USP is a chelating compound for removal of excess copper from the body.
Trientine hydrochloride, USP is available as 250 mg and 500 mg capsules for oral administration. Trientine hydrochloride capsules, USP contain gelatin, iron oxide black, iron oxide red, iron oxide yellow, potassium hydroxide, propylene glycol, shellac, stearic acid and titanium dioxide as inactive ingredients.
-
CLINICAL PHARMACOLOGY
Introduction
Wilson's disease (hepatolenticular degeneration) is an autosomal inherited metabolic defect resulting in an inability to maintain a near-zero balance of copper. Excess copper accumulates possibly because the liver lacks the mechanism to excrete free copper into the bile. Hepatocytes store excess copper but when their capacity is exceeded copper is released into the blood and is taken up into extrahepatic sites. This condition is treated with a low copper diet and the use of chelating agents that bind copper to facilitate its excretion from the body.
Clinical Summary
Forty-one patients (18 male and 23 female) between the ages of 6 and 54 with a diagnosis of Wilson's disease and who were intolerant of d-penicillamine were treated in two separate studies with trientine hydrochloride. The dosage varied from 450 to 2400 mg per day. The average dosage required to achieve an optimal clinical response varied between 1000 mg and 2000 mg per day. The mean duration of trientine hydrochloride therapy was 48.7 months (range 2 to 164 months). Thirty-four of the 41 patients improved, 4 had no change in clinical global response, 2 were lost to follow-up and one showed deterioration in clinical condition. One of the patients who improved while on therapy with trientine hydrochloride experienced a recurrence of the symptoms of systemic lupus erythematosus which had appeared originally during therapy with penicillamine. Therapy with trientine hydrochloride was discontinued. No other adverse reactions, except iron deficiency, were noted among any of these 41 patients.One investigator treated 13 patients with trientine hydrochloride following their development of intolerance to d-penicillamine. Retrospectively, he compared these patients to an additional group of 12 patients with Wilson's disease who were both tolerant of and controlled with d-penicillamine therapy, but who failed to continue any copper chelation therapy. The mean age at onset of disease of the latter group was 12 years as compared to 21 years for the former group. The trientine hydrochloride group received d-penicillamine for an average of 4 years as compared to an average of 10 years for the non- treated group.
Various laboratory parameters showed changes in favor of the patients treated with trientine hydrochloride. Free and total serum copper, SGOT, and serum bilirubin all showed mean increases over baseline in the untreated group which were significantly larger than with the patients treated with trientine hydrochloride. In the 13 patients treated with trientine hydrochloride, previous symptoms and signs relating to d-penicillamine intolerance disappeared in 8 patients, improved in 4 patients, and remained unchanged in one patient. The neurological status in the trientine hydrochloride group was unchanged or improved over baseline, whereas in the untreated group, 6 patients remained unchanged and 6 worsened. Kayser-Fleischer rings improved significantly during trientine hydrochloride treatment.
The clinical outcome of the two groups also differed markedly. Of the 13 patients on therapy with trientine hydrochloride (mean duration of therapy 4.1 years; range 1 to 13 years), all were alive at the data cutoff date, and in the non-treated group (mean years with no therapy 2.7 years; range 3 months to 9 years), 9 of the 12 died of hepatic disease.
Chelating Properties
Preclinical Studies
Studies in animals have shown that trientine hydrochloride has cupriuretic activities in both normal and copper-loaded rats. In general, the effects of trientine hydrochloride on urinary copper excretion are similar to those of equimolar doses of penicillamine, although in one study they were significantly smaller.Human Studies
Renal clearance studies were carried out with penicillamine and trientine hydrochloride on separate occasions in selected patients treated with penicillamine for at least one year. Six-hour excretion rates of copper were determined off treatment and after a single dose of 500 mg of penicillamine or 1.2 g of trientine hydrochloride. The mean urinary excretion rates of copper were as follows:
No. of Patients Single Dose Treatment Basal Excretion Rate (µg Cu + + /6hr) Test-dose ExcretionRate
(µg Cu+ + /6hr)6 Trientine,1.2 g 19 234 4 Penicillamine, 500 mg 17 320
In patients not previously treated with chelating agents, a similar comparison was made:No. of Patients Single Dose Treatment Basal Excretion Rate
(µg Cu++ /6hr)Test-dose Excretion
Rate (µg Cu++ /6hr)8 Trientine,1.2 g 71 1326 7 Penicillamine, 500 mg 68 1074
These results demonstrate that trientine hydrochloride is effective as a cupriuretic agent in patients with Wilson's disease although on a molar basis it appears to be less potent or less effective than penicillamine. Evidence from a radio-labelled copper study indicates that the different cupriuretic effect between these two drugs could be due to a difference in selectivity of the drugs for different copper pools within the body.
Pharmacokinetics
Data on the pharmacokinetics of trientine hydrochloride are not available. Dosage adjustment recommendations are based upon clinical use of the drug (see DOSAGE AND ADMINISTRATION). -
INDICATIONS AND USAGE
Trientine hydrochloride is indicated in the treatment of patients with Wilson's disease who are intolerant of penicillamine. Clinical experience with trientine hydrochloride is limited and alternate dosing regimens have not been well-characterized; all endpoints in determining an individual patient's dose have not been well defined. Trientine hydrochloride and penicillamine cannot be considered interchangeable. Trientine hydrochloride should be used when continued treatment with penicillamine is no longer possible because of intolerable or life endangering side effects.
Unlike penicillamine, trientine hydrochloride is not recommended in cystinuria or rheumatoid arthritis. The absence of a sulfhydryl moiety renders it incapable of binding cystine and, therefore, it is of no use in cystinuria. In 15 patients with rheumatoid arthritis, trientine hydrochloride was reported not to be effective in improving any clinical or biochemical parameter after 12 weeks of treatment.
Trientine hydrochloride is not indicated for treatment of biliary cirrhosis.
- CONTRAINDICATIONS
-
WARNINGS
Patient experience with trientine hydrochloride is limited (see CLINICAL PHARMACOLOGY). Patients receiving trientine hydrochloride capsules should remain under regular medical supervision throughout the period of drug administration. Patients (especially women) should be closely monitored for evidence of iron deficiency anemia.
-
PRECAUTIONS
General
There are no reports of hypersensitivity in patients who have been administered trientine hydrochloride for Wilson's disease. However, there have been reports of asthma, bronchitis and dermatitis occurring after prolonged environmental exposure in workers who use trientine hydrochloride as a hardener of epoxy resins. Patients should be observed closely for signs of possible hypersensitivity.
Information for Patients
Patients should be directed to take trientine hydrochloride capsules on an empty stomach, at least one hour before meals or two hours after meals and at least one hour apart from any other drug, food, or milk. The capsules should be swallowed whole with water and should not be opened or chewed. Because of the potential for contact dermatitis, any site of exposure to the capsule contents should be washed with water promptly. For the first month of treatment, the patient should have his temperature taken nightly, and he should be asked to report any symptom such as fever or skin eruption.
Laboratory Tests
The most reliable index for monitoring treatment is the determination of free copper in the serum, which equals the difference between quantitatively determined total copper and ceruloplasmin-copper. Adequately treated patients will usually have less than 10 mcg free copper/dL of serum.
Therapy may be monitored with a 24-hour urinary copper analysis periodically (i.e., every 6 to 12 months). Urine must be collected in copper-free glassware. Since a low copper diet should keep copper absorption down to less than one milligram a day, the patient probably will be in the desired state of negative copper balance if 0.5 to 1.0 milligram of copper is present in a 24-hour collection of urine.
Drug Interactions
In general, mineral supplements should not be given since they may block the absorption of trientine hydrochloride. However, iron deficiency may develop, especially in children and menstruating or pregnant women, or as a result of the low copper diet recommended for Wilson's disease. If necessary, iron may be given in short courses, but since iron and trientine hydrochloride each inhibit absorption of the other, two hours should elapse between administration of trientine hydrochloride capsules and iron.
It is important that trientine hydrochloride capsules be taken on an empty stomach, at least one hour before meals or two hours after meals and at least one hour apart from any other drug, food, or milk. This permits maximum absorption and reduces the likelihood of inactivation of the drug by metal binding in the gastrointestinal tract.Carcinogenesis, Mutagenesis, Impairment of Fertility
Data on carcinogenesis, mutagenesis, and impairment of fertility are not available.
Pregnancy
Trientine hydrochloride was teratogenic in rats at doses similar to the human dose. The frequencies of both resorptions and fetal abnormalities, including hemorrhage and edema, increased while fetal copper levels decreased when trientine hydrochloride was given in the maternal diets of rats. There are no adequate and well-controlled studies in pregnant women. Trientine hydrochloride should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when trientine hydrochloride is administered to a nursing mother.
Pediatric Use
Controlled studies of the safety and effectiveness of trientine hydrochloride in pediatric patients have not been conducted. It has been used clinically in pediatric patients as young as 6 years with no reported adverse experiences.
Geriatric Use
Clinical studies of trientine hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience is insufficient to determine differences in responses between the elderly and younger patients. In general, dose selection should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Clinical experience with trientine hydrochloride has been limited. The following adverse reactions have been reported in a clinical study in patients with Wilson's disease who were on therapy with trientine hydrochloride: iron deficiency, systemic lupus erythematosus (see CLINICAL PHARMACOLOGY). In addition, the following adverse reactions have been reported in marketed use: dystonia, muscular spasm, myasthenia gravis.
Trientine hydrochloride is not indicated for treatment of biliary cirrhosis, but in one study of 4 patients treated with trientine hydrochloride for primary biliary cirrhosis, the following adverse reactions were reported: heartburn; epigastric pain and tenderness; thickening, fissuring and flaking of the skin; hypochromic microcytic anemia; acute gastritis; aphthoid ulcers; abdominal pain; melena; anorexia; malaise; cramps; muscle pain; weakness; rhabdomyolysis. A causal relationship of these reactions to drug therapy could not be rejected or established.
To report SUSPECTED ADVERSE EVENTS, contact Rising Pharma Holdings, Inc. at 1-844-874-7464 or FDA at 1-800-FDA-1088 or http://www.fda.gov/ for voluntary reporting of adverse reactions.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Systemic evaluation of dose and/or interval between dose has not been done. However, on limited clinical experience, the recommended initial dose of trientine hydrochloride is 500-750 mg /day for pediatric patients and 750-1250 mg/day for adults given in divided doses two, three or four times daily. This may be increased to a maximum of 2000 mg/day for adults or 1500 mg/day for pediatric patients age 12 or under.
The daily dose of trientine hydrochloride should be increased only when the clinical response is not adequate or the concentration of free serum copper is persistently above 20 mcg/dL. Optimal long-term maintenance dosage should be determined at 6 to 12 month intervals (see PRECAUTIONS, Laboratory Tests).
It is important that trientine hydrochloride capsules be given on an empty stomach, at least one hour before meals or two hours after meals and at least one hour apart from any other drug, food, or milk. The capsules should be swallowed whole with water and should not be opened or chewed.
-
HOW SUPPLIED
Trientine Hydrochloride Capsule, USP 250 mg, are Size “1” hard gelatin capsule with brown opaque cap/brown opaque body, imprinted “C27” on cap with black ink and “250mg” on body with black ink filled with white to pale yellow powder. They are supplied as follows:
NDC: 16571-810-01 Bottles of 100 Capsules.
Trientine Hydrochloride Capsule, USP 500 mg, are Size “00” hard gelatin capsule with brown opaque cap/ brown opaque body, imprinted “C28” on cap with black ink and “500 mg” on body with black ink filled with white to pale yellow powder.
They are supplied as follows:
NDC: 16571-812-05 Bottles of 50 Capsules.
Storage
Keep container tightly closed. Store below 25°C (77°F). Store Trientine hydrochloride capsules in its original container.
Manufactured for:
Rising Pharma Holdings, Inc.
East Brunswick, NJ 08816Made in India
Neutral Code: 4323147/TS/DRUGS/2025Revised: 08/2025
PIR81205-01
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
CONTAINER LABEL
Rising Pharmaceuticals NDC: 16571-810-01
Trientine HCl Capsules, USP 250mg
100 Capsules Rx only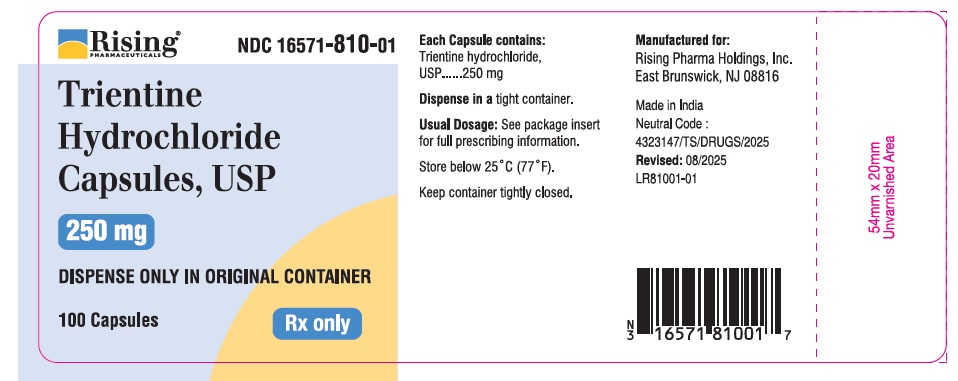
CARTON LABEL
Rising Pharmaceuticals NDC: 16571-810-01
Trientine HCl Capsules, USP 250 mg
100 Capsules Rx Only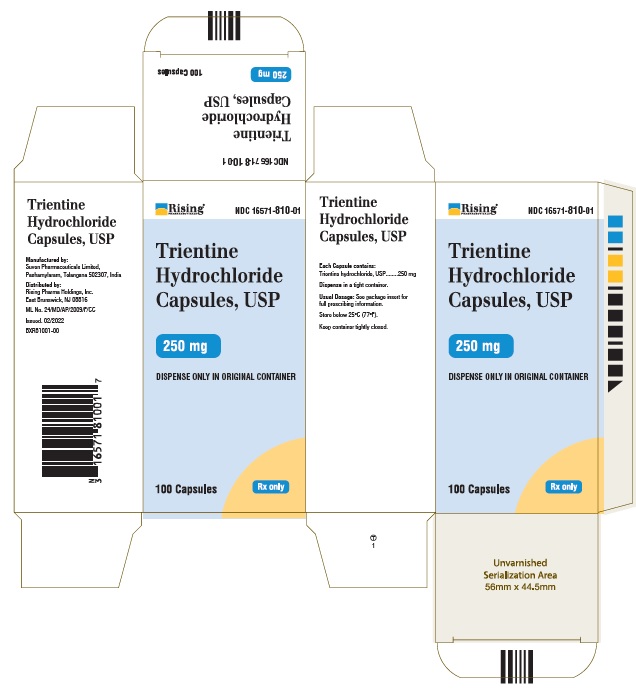
CONATINER LABEL
Rising Pharmaceuticals NDC: 16571-812-05
Trientine HCl Capsules, USP 500 mg
50 Capsules Rx Only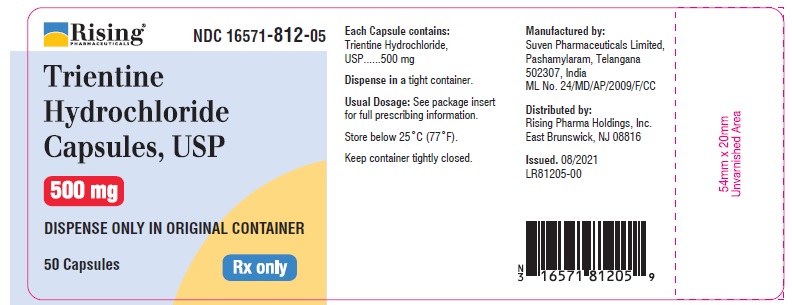
CARTON LABEL
Rising Pharmaceuticals NDC: 16571-812-05
Trientine HCl Capsules, USP 250 mg
100 Capsules Rx Only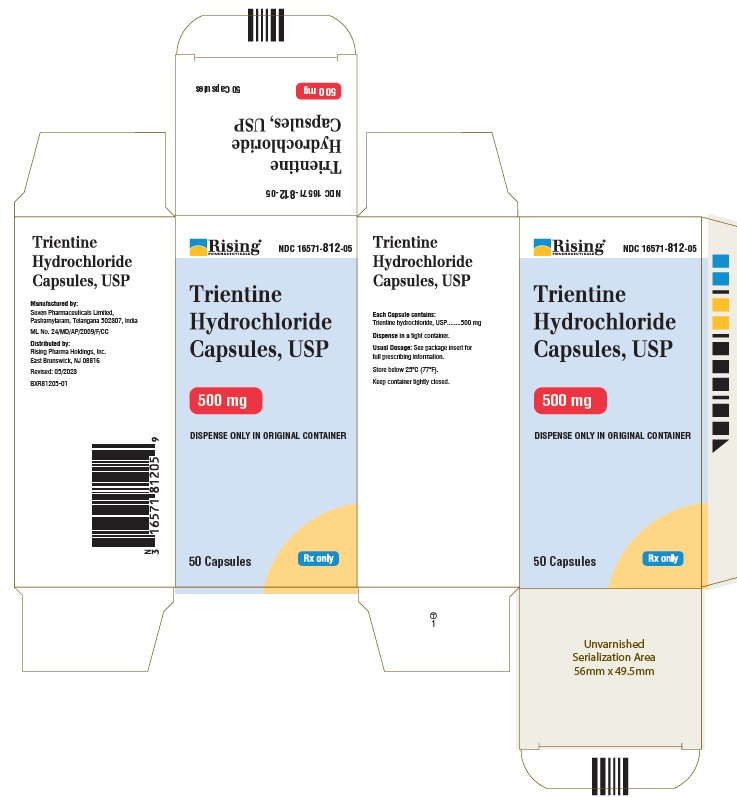
-
INGREDIENTS AND APPEARANCE
TRIENTINE HYDROCHLORIDE
trientine hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 16571-810 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIENTINE HYDROCHLORIDE (UNII: HC3NX54582) (TRIENTINE - UNII:SJ76Y07H5F) TRIENTINE HYDROCHLORIDE 250 mg Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BROWN (brown opaque) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code C27;250mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 16571-810-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212238 03/30/2021 TRIENTINE HYDROCHLORIDE
trientine hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 16571-812 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIENTINE HYDROCHLORIDE (UNII: HC3NX54582) (TRIENTINE - UNII:SJ76Y07H5F) TRIENTINE HYDROCHLORIDE 500 mg Inactive Ingredients Ingredient Name Strength FERRIC OXIDE RED (UNII: 1K09F3G675) GELATIN (UNII: 2G86QN327L) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color BROWN Score no score Shape CAPSULE Size 19mm Flavor Imprint Code C28;500 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 16571-812-05 50 in 1 BOTTLE; Type 0: Not a Combination Product 09/22/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212238 09/22/2023 Labeler - Rising Pharma Holdings, Inc. (116880195) Establishment Name Address ID/FEI Business Operations Cohance Lifesciences Limited 677604288 ANALYSIS(16571-810, 16571-812) , API MANUFACTURE(16571-812, 16571-810) , LABEL(16571-810, 16571-812) , MANUFACTURE(16571-810, 16571-812) , PACK(16571-810, 16571-812)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.