QUZYTTIR- cetirizine hydrochloride injection
QUZYTTIR by
Drug Labeling and Warnings
QUZYTTIR by is a Prescription medication manufactured, distributed, or labeled by TerSera Therapeutics LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use QUZYTTIR™ safely and effectively. See full prescribing information for QUZYTTIR™.
QUZYTTIR™ (cetirizine hydrochloride injection), for intravenous use
Initial U.S. Approval: 1995INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Injection: 10 mg/mL cetirizine hydrochloride (3)
CONTRAINDICATIONS
Known hypersensitivity to cetirizine hydrochloride or any of its ingredients, levocetirizine, or hydroxyzine (4)
WARNINGS AND PRECAUTIONS
Somnolence/Sedation: Exercise caution when driving a car or operating potentially dangerous machinery (5.1)
ADVERSE REACTIONS
The most common adverse reactions (incidence less than 1%) with QUZYTTIR are dysgeusia, headache, paresthesia, presyncope, dyspepsia, feeling hot, and hyperhidrosis.
Most common adverse reactions (incidence equal to or greater than 2%) with use of oral cetirizine hydrochloride are somnolence, fatigue, dry mouth, pharyngitis, and dizziness. (6)
To report SUSPECTED ADVERSE REACTIONS, call TerSera Therapeutics LLC at 1-844-334-4035 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Adults and adolescents 12 years of age and older
2.2 Children 6 to 11 years of age
2.3 Children 6 months to 5 years of age
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Somnolence/Sedation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
QUZYTTIR is a single use injectable product for intravenous administration only. The recommended dosage regimen is once every 24 hours as needed for treatment of acute urticaria. Administer QUZYTTIR as an intravenous push over a period of 1 to 2 minutes. QUZYTTIR is not recommended in pediatric patients less than 6 years of age with impaired renal or hepatic function [see Pediatric Use (8.4)].
2.1 Adults and adolescents 12 years of age and older
The recommended dosage is 10 mg administered by intravenous injection.
-
3 DOSAGE FORMS AND STRENGTHS
QUZYTTIR is a sterile, clear, colorless, non-pyrogenic, isotonic aqueous solution of cetirizine hydrochloride for intravenous injection; supplied in 2 mL size amber glass vials for single use. Each 2 mL size amber glass vial contains 1 mL drug solution with 10 mg cetirizine hydrochloride (equivalent to 8.42 mg of cetirizine).
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Somnolence/Sedation
In clinical trials with QUZYTTIR and cetirizine hydrochloride tablets, the occurrence of somnolence/sedation has been reported in some patients. Exercise due caution when driving a car or operating potentially dangerous machinery. Avoid concurrent use of QUZYTTIR with alcohol or other CNS depressants because additional reduction in alertness and additional impairment of CNS performance may occur.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reaction is described elsewhere in the labeling:
- Somnolence/Sedation [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Oral cetirizine hydrochloride
The following adverse reactions associated with the use of oral cetirizine hydrochloride were identified in clinical trials.
In clinical trials in patients 12 years and older the most common adverse reactions to oral cetirizine hydrochloride occurring with a 2% or greater incidence and greater than placebo were somnolence (14%), fatigue (6%), dry mouth (5%), pharyngitis (2%), and dizziness (2%). In clinical trials in children 6 to 11 years of age with oral cetirizine hydrochloride the most common adverse reactions occurring with a 2% or greater incidence and greater than placebo were headache, pharyngitis, abdominal pain, coughing, somnolence, diarrhea, epistaxis, bronchospasm, nausea, and vomiting. Somnolence appeared to be dose related. Adverse reactions reported in placebo-controlled trials with oral cetirizine hydrochloride in pediatric patients 2 to 5 years were qualitatively similar in nature and generally similar in frequency to those reported in trials with children 6 to 11 years of age. In placebo-controlled trials of pediatric patients 6 to 24 months of age, the incidences of adverse experiences were similar in the oral cetirizine hydrochloride and placebo treatment groups in each trial. In a trial of 1 week duration in children 6 to 11 months of age patients who received oral cetirizine hydrochloride exhibited greater irritability/fussiness than patients on placebo. In a trial of 18 months duration in patients 12 months and older, insomnia occurred more frequently in patients who received oral cetirizine hydrochloride compared to patients who received placebo.
QUZYTTIR
The safety data of QUZYTTIR was evaluated in a randomized, double-blind, single-dose, non-inferiority study comparing QUZYTTIR to intravenous diphenhydramine in 262 adults with acute urticaria.
The adverse reactions with QUZYTTIR occurred at an incidence of less than 1% and include: dyspepsia, feeling hot, dysgeusia, headache, paresthesia, presyncope, and hyperhidrosis.
An additional randomized, double-blind, single dose study was conducted in 33 adults which showed similar safety results.
Sedation
Subject-rated sedation scores were assessed at baseline, 1 hr, and/or 2 hrs, and/or "Readiness for Discharge”. Sedation was rated on a 0 to 3 scale (0 = none, to 3 = severe) with lower sedation scores indicating less sedation. Subjects in the QUZYTTIR treatment group reported less sedation at all time points compared to subjects treated with diphenhydramine.
-
7 DRUG INTERACTIONS
No clinically significant drug interactions with oral cetirizine hydrochloride, the active ingredient in QUZYTTIR, have been found with theophylline at a low dose, azithromycin, pseudoephedrine, ketoconazole, or erythromycin. There was a small decrease in the clearance of oral cetirizine hydrochloride caused by a 400-mg dose of theophylline; it is possible that larger theophylline doses could have a greater effect [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies in pregnant women with cetirizine hydrochloride the active ingredient in QUZYTTIR. In animal reproduction studies, there was no evidence of fetal harm with administration of cetirizine hydrochloride by the oral route to pregnant mice, rats, and rabbits, during the period of organogenesis, at doses that were 45 times and higher than the maximum recommended human dose (MRHD) in adults. In rats treated during late gestation and the lactation period, cetirizine hydrochloride had no effects on pup development at oral doses up to approximately 30 times the MRHD in adults. In mice treated during late gestation and the lactation period, cetirizine hydrochloride administered by the oral route to the dams had no effects on pup development at a dose that was approximately 10 times the MRHD in adults; however, lower pup weight gain during lactation was observed at a dose that was 45 times the MRHD in adults (See Data). The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20% respectively.
Data
Animal Data: In embryofetal development studies conducted in mice, rats, and rabbits, cetirizine hydrochloride, administered during the period of organogenesis, was not teratogenic at doses up to 45, 220, and 260 times the MRHD, respectively (on a mg/m2 basis with maternal oral doses up to 96, 225, and 135 mg/kg).
In a prenatal and postnatal development (PPND) study conducted in mice, cetirizine hydrochloride was administered at oral doses up to 96 mg/kg/day from gestation day 15 through lactation day 21. Cetirizine hydrochloride lowered pup body weight gain during lactation at an oral dose in dams that was approximately 45 times the MRHD (on a mg/m2 basis with a maternal oral dose of 96 mg/kg/day); however, there were no effects on pup weight gain at an oral dose in dams that was approximately 10 times the MRHD (on a mg/m2 basis with a maternal oral dose of 24 mg/kg/day). In a PPND study conducted in rats, cetirizine hydrochloride was administered at oral doses up to 180 mg/kg/day from gestation day 17 to lactation day 22. Cetirizine hydrochloride did not have any adverse effects on rat dams or offspring development at doses up to approximately 30 times the MRHD (on a mg/m2 basis with a maternal oral dose of 30 mg/kg/day). Cetirizine hydrochloride caused excessive maternal toxicity at an oral dose in dams that was approximately 180 times the MRHD (on a mg/m 2 basis with a maternal oral dose of 180 mg/kg/day).
8.2 Lactation
Risk Summary
Cetirizine hydrochloride has been reported to be present in human breast milk. In mice and beagle dogs, studies indicated that cetirizine hydrochloride was excreted in milk (See Data). When a drug is present in animal milk, it is likely the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for QUZYTTIR and any potential adverse effects on the breastfed child from QUZYTTIR or from the underlying maternal condition.
Data
Animal Data: Cetirizine hydrochloride was detected in the milk of mice. No adverse developmental effects on pups were seen when cetirizine hydrochloride was administered orally to dams during lactation at a dose that was approximately 10 times the MRHD in adults [See Use in Specific Populations (8.1)]. Studies in beagle dogs indicated that approximately 3% of the dose of cetirizine hydrochloride was excreted in milk. The concentration of drug in animal milk does not necessarily predict the concentration of drug in human milk.
8.4 Pediatric Use
The safety and efficacy of QUZYTTIR have been established in patients 6 months to 17 years of age. The efficacy of QUZYTTIR for the treatment of acute urticaria down to 6 months of age is based on extrapolation of the efficacy of QUZYTTIR in adults with acute urticaria [See Clinical Studies (14)] and supported by pharmacokinetic data with oral cetirizine hydrochloride (the active ingredient in QUZYTTIR) in patients 6 months to 17 years of age. Based upon the known PK profile of oral cetirizine hydrochloride, the exposure of IV cetirizine hydrochloride in pediatric patients (6 months to 17 years of age) is expected to be similar to the exposure of IV cetirizine hydrochloride in adults at the labeled doses. Extrapolation of efficacy is based on the likelihood that the disease course, pathophysiology and the drug's effect are similar between these two populations.
The safety of QUZYTTIR in children 6 months to 17 years of age is supported by safety information from placebo-controlled clinical trials with oral cetirizine hydrochloride in patients 6 months of age and older [see Adverse Reactions (6)]. QUZYTTIR demonstrates a higher Cmax compared to oral cetirizine hydrochloride in adults [See Clinical Pharmacology (12.3)]. As QUZYTTIR is indicated for an acute condition administered in a medically supervised setting, the safety for higher Cmax in children 6 months to less than 18 years of age is supported by the safety data from the clinical trial with IV cetirizine hydrochloride in adults [see Adverse Reactions (6)] and available safety information from pediatric overdose cases.
Because of the absence of pharmacokinetic and safety information for cetirizine hydrochloride in children below 6 years of age with impaired renal or hepatic function, the use of QUZYTTIR in this impaired patient population is not recommended [see Dosage And Administration (2)].
The safety and efficacy of QUZYTTIR in patients less than 6 months of age has not been established.
8.5 Geriatric Use
In clinical trials with QUZYTTIR, 18 patients were 65 years and older, and 6 patients were 75 years and older. No overall differences in safety were observed between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out. In clinical trials with cetirizine hydrochloride oral tablets, 186 patients were 65 years and older, and 39 patients were 75 years and older. No overall differences in safety were observed between these patients and younger patients.
With regard to efficacy, the clinical trials with cetirizine hydrochloride oral tablets and QUZYTTIR did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently than younger patients.
8.6 Hepatic Impairment
No dosage adjustment is required in patients with hepatic impairment. Monitor for antihistaminic side effects in this patient population [See Clinical Pharmacology (12.3)].
8.7 Renal Impairment
No dosage adjustment is required in patients with moderate and severe renal impairment and in patients on dialysis. Monitor for antihistaminic side effects in this patient population [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Cases of adult and pediatric patients with overdoses of only oral cetirizine hydrochloride have been reported, some of which resulted in adverse reactions. Adult overdose cases involved patients 18 to 81 years of age receiving oral cetirizine hydrochloride doses of 70 mg to 800 mg (7 to 80 times the maximum recommended dosage of 10 mg/day in adults). The most commonly reported adverse reactions were somnolence and fatigue. Other reported adverse reactions included tachycardia, abdominal pain, nausea, and vomiting. Pediatric overdose cases involved patients 18 months to 15 years of age receiving oral cetirizine hydrochloride doses of 90 mg to 300 mg (9 to 72 times the maximum age recommended dose). The adverse reactions reported included: somnolence, difficulty walking, agitation/irritability, hard to swallow/articulate clearly, tachycardia, vomiting, mydriasis, and elevated creatinine phosphokinase.
If overdose with QUZYTTIR occurs, treatment should be symptomatic or supportive, taking into account any concomitantly ingested medications. There is no known specific antidote to cetirizine hydrochloride. Cetirizine hydrochloride is not effectively removed by dialysis, and dialysis will be ineffective unless a dialyzable agent has been concomitantly ingested.
-
11 DESCRIPTION
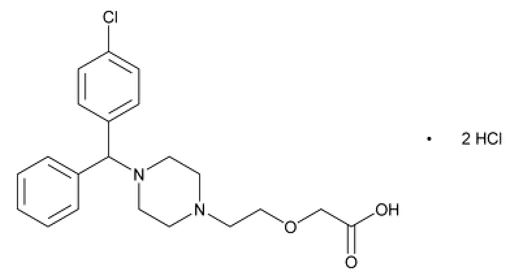
Cetirizine hydrochloride, the active component of QUZYTTIR, is a selective histamine-1 (H1) receptor antagonist. The chemical name is (±) –[2-[4-[(4-chlorophenyl)phenylmethyl]-1- piperzinyl] ethoxy]acetic acid, dihydrochloride. Cetirizine hydrochloride is a racemic compound with an empirical formula of C21H25ClN2O32HCl. The molecular weight is 461.82 and the chemical structure is shown below:
Cetirizine hydrochloride is a white, crystalline powder and is water soluble. QUZYTTIR is a sterile, clear, colorless, non-pyrogenic, isotonic solution for intravenous injection. Each mL of QUZYTTIR injection contains 10 mg cetirizine hydrochloride (equivalent to cetirizine 8.42 mg) and 6.5 mg sodium chloride, USP to adjust solution tonicity, in water for injection, USP. QUZYTTIR is supplied in 2 mL size amber glass vials for single use. Each 2 mL size amber glass vial contains 1 mL drug solution with 10 mg cetirizine hydrochloride (10 mg/mL). QUZYTTIR's pH is maintained between 4.5 to 6.5 with sodium hydroxide and/or hydrochloric acid as needed. The osmolality of QUZYTTIR injection is between 255 to 340 mOsmol.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Cetirizine hydrochloride, a human metabolite of hydroxyzine, is an antihistamine; its principal effects are mediated via selective inhibition of peripheral H1-receptors. The antihistaminic activity of cetirizine hydrochloride has been clearly documented in a variety of animal and human models. In vivo and ex vivo animal models have shown negligible anticholinergic and antiserotonergic activity. In clinical studies however, dry mouth was more common with cetirizine hydrochloride than with placebo. In vitro receptor binding studies have shown no measurable affinity for receptors other than H1 receptors.
12.2 Pharmacodynamics
Studies in 69 adult normal volunteers (aged 20 to 61 years) showed that cetirizine hydrochloride oral tablets at doses of 5 and 10 mg strongly inhibited the skin wheal and flare caused by the intradermal injection of histamine. The onset of this activity after a single 10-mg oral dose occurred within 20 minutes in 50% of subjects and within one hour in 95% of subjects; this activity persisted for at least 24 hours. Cetirizine hydrochloride tablets at doses of 5 and 10 mg also strongly inhibited the wheal and flare caused by intradermal injection of histamine in 19 pediatric volunteers (aged 5 to 12 years) and the activity persisted for at least 24 hours. In a 35-day study in children aged 5 to 12, no tolerance to the antihistaminic (suppression of wheal and flare response) effects of cetirizine hydrochloride oral tablets was found. In 10 infants 7 to 25 months of age who received 4 to 9 days of cetirizine hydrochloride in an oral solution (0.25 mg/kg bid), there was a 90% inhibition of histamine-induced (10 mg/mL) cutaneous wheal and 87% inhibition of the flare 12 hours after administration of the last dose. The clinical relevance of this suppression of histamine-induced wheal and flare response on skin testing is unknown.
The effects of intradermal injection of various other mediators or histamine releasers were also inhibited by oral cetirizine hydrochloride, as was response to a cold challenge in patients with cold-induced urticaria. In mildly asthmatic subjects, cetirizine hydrochloride oral tablets at 5 to 20 mg blocked bronchoconstriction due to nebulized histamine, with virtually total blockade after a 20-mg dose. In studies conducted for up to 12 hours following cutaneous antigen challenge, the late phase recruitment of eosinophils, neutrophils and basophils, components of the allergic inflammatory response, was inhibited by cetirizine hydrochloride oral tablets at a dose of 20 mg.
Cardiac Electrophysiology
In four clinical studies in healthy adult males, no clinically significant mean increases in QTc were observed in subjects treated with cetirizine hydrochloride oral tablets. In the first study, a placebo-controlled crossover trial, cetirizine hydrochloride oral tablets were given at doses up to 60 mg per day, 6 times the maximum clinical dose, for 1 week, and no significant mean QTc prolongation occurred. In the second study, a crossover trial, cetirizine hydrochloride oral tablets 20 mg and erythromycin (500 mg every 8 hours) were given alone and in combination. There was no significant effect on QTc with the combination or with cetirizine hydrochloride alone. In the third trial, also a crossover study, cetirizine hydrochloride oral tablet 20 mg and ketoconazole (400 mg per day) were given alone and in combination. Cetirizine caused a mean increase in QTc of 9.1 msec from baseline after 10 days of therapy. Ketoconazole also increased QTc by 8.3 msec. The combination caused an increase of 17.4 msec, equal to the sum of the individual effects. Thus, there was no significant drug interaction on QTc with the combination of cetirizine and ketoconazole. In the fourth study, a placebo-controlled parallel trial, cetirizine hydrochloride oral tablet 20 mg was given alone or in combination with azithromycin (500 mg as a single dose on the first day followed by 250 mg once daily). There was no significant increase in QTc with cetirizine hydrochloride 20 mg alone or in combination with azithromycin.
In a four-week clinical trial in pediatric patients aged 6 to 11 years, results of randomly obtained ECG measurements before treatment and after 2 weeks of treatment showed that cetirizine hydrochloride oral tablet 5 or 10 mg did not increase QTc versus placebo. In a one week clinical trial (N=86) of cetirizine hydrochloride oral syrup (0.25 mg/kg bid) compared with placebo in pediatric patients 6 to 11 months of age, ECG measurements taken within 3 hours of the last dose did not show any ECG abnormalities or increases in QTc interval in either group compared to baseline assessments. Data from other studies where cetirizine hydrochloride oral was administered to patients 6-23 months of age were consistent with the findings in this study.
The effects of cetirizine hydrochloride on the QTc interval at doses higher than 10 mg have not been studied in children less than 12 years of age.
12.3 Pharmacokinetics
In a single dose crossover study in healthy volunteers under fasting conditions, cetirizine reached a mean Cmax of 495 ng/mL and 1344 ng/mL following single dose intravenous (IV) administration of 5 mg and 10 mg, respectively, injected over a period of 1 to 1.5 minutes. Peak concentrations were reached at 0.06 hour (range 0.03 to 0.07 hour) and 0.03 hour (range 0.03 to 2.00 hour) for cetirizine hydrochloride 5 mg and 10 mg IV injection, respectively. The mean systemic exposure (AUC0-inf) for cetirizine hydrochloride 5 mg and 10 mg IV injection was 1318 ng·hr/mL and 2746 ng·hr/mL, respectively. The AUC0-inf for cetirizine hydrochloride 10 mg oral tablet in the study was 2651 ng·hr/mL.
Absorption
Following oral administration of tablets or syrup in adults, cetirizine was rapidly absorbed with a time to maximum concentration (Tmax) of approximately 1 hour. When healthy volunteers were administered multiple doses of cetirizine hydrochloride (10 mg oral tablets once daily for 10 days), a mean peak plasma concentration (Cmax) of 311 ng/mL was observed and there was no accumulation. Cetirizine pharmacokinetics were linear for oral doses ranging from 5 to 60 mg. Food had no effect on the extent of cetirizine exposure (AUC) but Tmax was delayed by 1.7 hours and Cmax was decreased by 23% in the presence of food when cetirizine hydrochloride was administered orally.
Distribution
The mean plasma protein binding of cetirizine is 93%, independent of concentration in the range of 25-1000 ng/mL, which includes the therapeutic plasma levels observed.
Elimination
The mean elimination half-life in 146 healthy volunteers across multiple pharmacokinetic studies was 8.3 hours and the apparent total body clearance for cetirizine was approximately 53 mL/min.
Metabolism
Cetirizine is metabolized to a limited extent by oxidative O-dealkylation to a metabolite with negligible antihistaminic activity. The enzyme or enzymes responsible for this metabolism have not been identified.
Excretion
A mass balance study in 6 healthy male volunteers indicated that 70% of the administered radioactivity was recovered in the urine and 10% in the feces. Approximately 50% of the radioactivity was identified in the urine as unchanged drug. Most of the rapid increase in peak plasma radioactivity was associated with parent drug, suggesting a low degree of first-pass metabolism.
Specific Populations
Geriatric Patients: Following a single, 10-mg oral dose, the elimination half-life was prolonged by 50% and the apparent total body clearance was 40% lower in 16 geriatric subjects with a mean age of 77 years compared to 14 adult subjects with a mean age of 53 years. The decrease in cetirizine clearance in these elderly volunteers may be related to decreased renal function.
Pediatric Patients: When pediatric patients aged 7 to 12 years received a single, 5-mg oral cetirizine hydrochloride capsule, the mean Cmax was 275 ng/mL. Based on cross-study comparisons, the weight-normalized, apparent total body clearance was 33% greater and the elimination half-life was 33% shorter in this pediatric population than in adults. In pediatric patients aged 2 to 5 years who received 5 mg oral tablets of cetirizine hydrochloride, the mean Cmax was 660 ng/mL. Based on cross-study comparisons, the weight-normalized apparent total body clearance was 81 to 111% greater and the elimination half-life was 33 to 41% shorter in this pediatric population than in adults. In pediatric patients aged 6 to 23 months who received a single dose of 0.25 mg/kg cetirizine hydrochloride oral solution (mean dose 2.3 mg), the mean Cmax was 390 ng/mL. Based on cross-study comparisons, the weight-normalized, apparent total body clearance was 304% greater and the elimination half-life was 63% shorter in this pediatric population compared to adults. The average AUC (0-t) in children 6 months to less than 2 years of age receiving the maximum dose of cetirizine hydrochloride oral solution (2.5 mg twice a day) is expected to be two-fold higher than that observed in adults receiving a dose of 10 mg cetirizine hydrochloride oral tablets once a day.
Male and Female Patients: The effect of gender of cetirizine pharmacokinetics has not been adequately studied.
Racial or Ethnic Groups: No race-related difference in the kinetics of cetirizine has been observed.
Patients with Renal Impairment: The kinetics of cetirizine were studied following multiple, oral, 10-mg daily doses of cetirizine hydrochloride for 7 days in 7 normal volunteers (creatine clearance 89-128 mL/min), 8 patients with mild renal function impairment (creatinine clearance 42-77 mL/min) and 7 patients with moderate renal function impairment (creatine clearance 11-31 mL/min). The pharmacokinetics of oral cetirizine were similar in patients with mild impairment and normal volunteers. Moderately impaired patients had a 3-fold increase in half-life and a 70% decrease in clearance compared to normal volunteers. Patients on hemodialysis (n =5) given a single, 10-mg oral dose of cetirizine hydrochloride had a 3-fold increase in half-life and a 70% decrease in clearance compared to normal volunteers. Less than 10% of the administered dose was removed during the single dialysis session.
The pharmacokinetics of IV cetirizine has not been evaluated in patients with renal impairment.
Patients with Hepatic Impairment: Sixteen patients with chronic liver diseases (hepatocellular, cholestatics, and biliary cirrhosis), given 10 or 20 mg of cetirizine hydrochloride as a single oral dose had a 50% increase in half-life along with a corresponding 40% decrease in clearance compared to 16 healthy subjects.
Drug Interaction Studies
No interactions were observed in pharmacokinetic interaction studies conducted with oral cetirizine hydrochloride and pseudoephedrine, antipyrine, ketoconazole, erythromycin and azithromycin. In a multiple dose study of theophylline (400 mg once daily for 3 days) and cetirizine hydrochloride (20 mg oral tablets once daily for 3 days), a 16% decrease in the clearance of cetirizine was observed. The disposition of theophylline was not altered by concomitant cetirizine hydrochloride administration.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 2-year carcinogenicity study in rats, cetirizine hydrochloride was not carcinogenic at dietary doses up to 20 mg/kg (approximately 20, 10, 25, and 6 times the MRHDs in adults, children 6 to 11 years of age, children 2 to 5 years of age, and children 6 months to less than 2 years of age, respectively, on a mg/m2 basis). In a 2-year carcinogenicity study in mice, cetirizine hydrochloride caused an increased incidence of benign hepatic tumors in males at a dietary dose of 16 mg/kg (approximately 8, 4, 9, and 2 times the MRHDs in adults, children 6 to 11 years of age, children 2 to 5 years of age, and children 6 months to less than 2 years of age, respectively, on a mg/m2 basis). No increased incidence of benign hepatic tumors was observed in mice at a dietary dose of 4 mg/kg (approximately 2, 1, 2, and 0.5 times the MRHDs in adults, children 6 to 11 years of age, children 2 to 5 years of age, and children 6 months to less than 2 years of age, respectively, on a mg/m 2 basis). The clinical significance of these findings during long-term use of QUZYTTIR is not known.
-
14 CLINICAL STUDIES
The safety and efficacy of QUZYTTIR for the treatment of acute urticaria was demonstrated in a randomized, active-controlled, double-blind, single dose, multicenter (US and Canada), parallel group trial in 262 patients 18 years of age and older presenting to Emergency Departments or Urgent care Centers (NCT02935699). Subjects were treated with 10 mg of QUZYTTIR or 50 mg diphenhydramine injection. Patients with acute urticaria with or without other diseases were enrolled, including patients with concomitant angioedema. The majority of the patients were Caucasian (48%) and female (63%) with a mean age of 39 years.
The primary efficacy endpoint was the change from baseline in patient-rated pruritus score assessed 2 hrs post treatment for the intent-to-treat (ITT) population. Pruritus was graded on a severity score of 0 to 3 with 0 = no pruritus, 1 = mild, 2 =moderate, and 3 = severe. The trial was non-inferiority design with the pre-specified non-inferiority margin of 0.50 for the difference between treatment groups. Two key secondary efficacy outcome measures: (i) the need to return to any ED or clinic after patient discharge, and (ii) time spent at the treatment center (time from treatment administration to readiness for discharge) were adjusted for multiplicity.
Result for the change from baseline in the pruritus scores are shown in Table 1. The difference between treatment groups excluded the pre-specified non-inferiority margin, i.e. the lower bound of the 95% confidence interval for the difference of diphenhydramine minus QUZYTTIR did not include – 0.50. The primary efficacy data are presented in Table 1.
Table 1. Primary Efficacy Endpoint: Patient-rated Pruritus Score Change from Baseline at 2 hrs (using LOCF); ITT population LOCF: last observation carried forward; ITT: intent-to-treat
*Since the lower bound of the 95% CI for the treatment difference was > -0.50, effectiveness of QUZYTTIR injection was demonstrated to be non-inferior to the effectiveness of diphenhydramine injection. The treatment difference and 95% CI were obtained from a generalized linear mixed-effects model. The model consisted of the change from baseline at 2 hours as the dependent variable and site, treatment and site-by-treatment interaction as the fixed effect.
Diphenhydramine injection 50 mg
(N = 135)QUZYTTIR injection 10 mg
(N = 127)Adjusted Difference between treatment (95% CI) Baseline: mean (SD) 2.19 (0.748) 2.20 (0.727) Change from Baseline: mean (SD) -1.50 (0.984) -1.61 (0.944) 0.06 (-0.28, 0.40)* Additionally, in this trial the proportion of patients returning to any emergency department or clinic was lower in the QUZYTTIR treatment group (6%) compared to the diphenhydramine treatment group (14%), and the time spent in the treatment center (hours spent reported as mean (SD) was shorter in the QUZYTTIR treatment group (1.7 (0.9)) compared to the diphenhydramine treatment group (2.1 (1.1)).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
QUZYTTIR (cetirizine hydrochloride injection), 10 mg/mL, pH between 4.5 to 6.5, is supplied as a sterile, clear, colorless, non-pyrogenic isotonic aqueous solution in single-use 2 mL amber glass vials. The following packaging configuration is available:
- NDC: 70720-100-01: 10 mg/mL cetirizine hydrochloride single-use vial
- NDC: 70720-100-10: Carton containing 1 single-use vial (10 mg/mL cetirizine hydrochloride)
- NDC: 70720-100-25: Carton containing 25 single-use vials (10 mg/mL cetirizine hydrochloride)
-
17 PATIENT COUNSELING INFORMATION
Somnolence/Sedation
Inform patients that somnolence has occurred with administration of QUZYTTIR. Instruct patients to exercise caution when driving a car or operating potentially dangerous machinery. Instruct patients to avoid use of alcohol or other CNS depressants after they have received treatment with QUZYTTIR because additional reduction in alertness and additional impairment of CNS performance might occur [see Warnings and Precautions (5.1)].
QUZYTTIR™ is a trademark of TerSera Therapeutics LLC or its affiliates.
Manufactured by: Pfizer Rocky Mount, NC 27801
Distributed by: TerSera Therapeutics LLC, Deerfield, IL 60015
©2020 TerSera Therapeutics LLCU.S. Patent Numbers:
9,119,771; 8,263,581; 8,513,259; 8,314,083; 9,180,090 -
PRINCIPAL DISPLAY PANEL
Principal Display Panel – Carton Label
NDC: 70720-100-25
Quzyttir™
Cetirizine HCl Injection
10 mg/mL
For Intravenous Use Only
Each 1 mL vial contains Cetirizine Hydrochloride 10 mg (equivalent to
8.42 mg of Cetirizine) and sodium chloride for tonicity adjustmentSingle-Dose Vial - Discard Unused Portion
Carton of 25
1 mL Single-dose vials
Rx only
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – Carton Label
NDC: 70720-100-10 Rx only
Quzyttir™
Cetirizine HCl Injection
10 mg/mL
For Intravenous Use Only
Single-Dose Vial - Discard Unused Portion
1 mL Single-Dose Vial
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – Vial Label
NDC: 70720-100-01
Quzyttir™
Cetirizine HCl
Injection10 mg/mL
For Intravenous Use Only
1 mL
Rx Only
Each 1 mL vial contains
Cetirizine Hydrochloride
10 mg (equivalent to
8.42 mg of Cetirizine)
and sodium chloride for
tonicity adjustmentSingle-Dose Vial -
Discard Unused PortionDistributed by:
TerSera Therapeutics LLC -
INGREDIENTS AND APPEARANCE
QUZYTTIR
cetirizine hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70720-100 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength cetirizine hydrochloride (UNII: 64O047KTOA) (cetirizine - UNII:YO7261ME24) cetirizine hydrochloride 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) Sodium Hydroxide (UNII: 55X04QC32I) Hydrochloric Acid (UNII: QTT17582CB) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70720-100-25 25 in 1 CARTON 11/13/2019 1 NDC: 70720-100-01 1 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 2 NDC: 70720-100-10 1 in 1 CARTON 04/02/2020 2 NDC: 70720-100-01 1 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211415 11/13/2019 Labeler - TerSera Therapeutics LLC (080226115)
Trademark Results [QUZYTTIR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 QUZYTTIR 88153622 not registered Live/Pending |
JDP THERAPEUTICS LLC 2018-10-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.