ONAPGO- apomorphine hydrochloride injection, solution
ONAPGO by
Drug Labeling and Warnings
ONAPGO by is a Prescription medication manufactured, distributed, or labeled by MDD US Operations, LLC, a subsidiary of Supernus Pharmaceuticals, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ONAPGO™ safely and effectively. See full prescribing information for ONAPGO™.
ONAPGO™ (apomorphine hydrochloride) injection, for subcutaneous use
Initial U.S. Approval: 2004INDICATIONS AND USAGE
ONAPGO is a dopaminergic agonist indicated for the treatment of motor fluctuations in adults with advanced Parkinson's disease. ( 1)
DOSAGE AND ADMINISTRATION
- For subcutaneous use by infusion only. ( 2.1)
- Maximum recommended total daily dosage of ONAPGO, including the continuous dosage and any extra dose(s), is 98 mg generally administered over the waking day (e.g., 16 hours). ( 2.3)
- The recommended initial continuous dosage is 1 mg/hr with a maximum of 6 mg/hr for up to 16 hours per day. ( 2.3)
- The extra dose may be titrated in increments of 0.5 mg or 1 mg based on clinical response and tolerability. Subsequent extra doses are between 0.5 mg and 2 mg, with at least 3 hours between extra doses and a maximum of 3 extra doses per day. ( 2.3)
- Treatment with trimethobenzamide is recommended, starting 3 days prior to the first dose of ONAPGO, and should only be continued as long as necessary to control nausea and vomiting, and generally no longer than two months. ( 2.2, 5.2, 6.1, 17)
- ONAPGO is not substitutable for apomorphine products intended for intermittent use. ( 2.3)
- For patients with mild or moderate renal impairment, the recommended initial extra dose is 0.5 mg to 1 mg and should not exceed 1 mg. ( 2.5)
DOSAGE FORMS AND STRENGTHS
Injection: 98 mg/20 mL (4.9 mg/mL) of apomorphine hydrochloride in single-dose cartridges. ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Subcutaneous use only; thrombus formation and pulmonary embolism have followed intravenous administration of ONAPGO. ( 5.1)
- May cause nausea and vomiting. ( 2.2, 5.2)
- Falling asleep during activities of daily living and daytime somnolence may occur. ( 5.3)
- May cause hypotension/orthostatic hypotension and syncope may occur. ( 5.4)
- May cause or increase the risk of falls. ( 5.5)
- May cause infusion site reactions and infections. Monitor and change infusion site every day. ( 2.4, 5.6)
- May cause hallucinations and psychotic-like behavior. ( 5.7)
- May cause dyskinesia or exacerbate pre-existing dyskinesia. ( 5.8)
- May cause hemolytic anemia. ( 5.9)
- May cause impulse control/ compulsive and impulsive behaviors. Consider dose reductions or stopping ONAPGO. ( 5.10)
- May cause cardiac events. ( 5.11)
- May prolong QTc and cause torsades de pointes or sudden death. ( 5.12)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥10% on ONAPGO and at least twice the rate of placebo) were infusion site nodule, nausea, somnolence, infusion site erythema, dyskinesia, headache, and insomnia. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact MDD US Operations, LLC, a subsidiary of Supernus Pharmaceuticals, Inc. at toll-free number 1-833-366-2746 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Information
2.2 Premedication and Concomitant Medication
2.3 Recommended Dosage
2.4 Preparation and Administration Instructions
2.5 Recommended Dosage in Patients with Renal Impairment
2.6 Discontinuation of ONAPGO
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Adverse Reactions After Intravenous Administration
5.2 Nausea and Vomiting
5.3 Falling Asleep During Activities of Daily Living and Somnolence
5.4 Syncope/Hypotension/Orthostatic Hypotension
5.5 Falls
5.6 Infusion Site Reactions and Infections
5.7 Hallucinations/Psychotic-Like Behavior
5.8 Dyskinesia
5.9 Hemolytic Anemia
5.10 Impulse Control/Compulsive Behaviors
5.11 Cardiac Events
5.12 QTc Prolongation and Potential for Proarrhythmic Effects
5.13 Hypersensitivity
5.14 Fibrotic Complications
5.15 Priapism
5.16 Retinal Pathology in Albino Rats
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 5HT 3Antagonists
7.2 Antihypertensive Medications and Vasodilators
7.3 Alcohol
7.4 Dopamine Antagonists
7.5 Drugs Prolonging the QT/QTc Interval
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Information
- ONAPGO is indicated for subcutaneous use by infusion only [see Warnings & Precautions (5.1)] .
- Patients selected for treatment with ONAPGO should be capable of understanding and trained on using the delivery system, either themselves or with the assistance of a caregiver [see Healthcare Provider Instructions for Use and Patient Instructions for Use].
- ONAPGO initiation and dose titrations should be done under medical supervision.
- The prescribed dose of ONAPGO should be expressed in "mg/hr" for the continuous dosage and "mg" for an extra dose.
2.2 Premedication and Concomitant Medication
Because of the incidence of nausea and vomiting with ONAPGO, it is recommended that treatment with trimethobenzamide 300 mg three times a day start 3 days prior to the initial dose of ONAPGO [see Adverse Reactions (6.1)and Warnings and Precautions (5.2)] . Alternatively, consider starting ONAPGO therapy, without antiemetics, at 1 mg/hour and titrate based upon effectiveness and tolerance.
If trimethobenzamide is used, it should be continued only as long as necessary to control nausea and vomiting, and generally no longer than two months after initiation of treatment with ONAPGO, as trimethobenzamide increases the incidence of somnolence, dizziness, and falls in patients treated with ONAPGO [see Warnings and Precautions (5.2)] .
Based on reports of profound hypotension and loss of consciousness when apomorphine was administered with ondansetron, the concomitant use of apomorphine with drugs of the 5HT 3antagonist class including antiemetics (e.g., ondansetron, granisetron, dolasetron, palonosetron) and alosetron are contraindicated [see Contraindications (4)] .
After starting ONAPGO, adjustment of concomitant anti-Parkinson's disease medications may be necessary.
2.3 Recommended Dosage
ONAPGO (apomorphine hydrochloride) is administered as a subcutaneous infusion with the ONAPGO pump [see Dosage and Administration (2.3), Healthcare Provider Instructions for Use, and Patient Instructions for Use].
The daily dosage is determined by individualized patient titration and is composed of a continuous dosage and as needed extra dose(s).
The maximum recommended total daily dosage of ONAPGO, including the continuous dosage and any extra dose(s), is 98 mg per day, generally administered over the waking day (e.g., 16 hours).
Continuous Dosage
The recommended initial continuous dosage (continuous infusion) of ONAPGO is 1 mg/hr. Titrate the continuous dosage, as needed, in 0.5 mg/hr to 1 mg/hr increments. Dose adjustments may be made daily, or at longer intervals, through the titration process. Patients in clinical studies used a mean of 4 mg/hr of ONAPGO. The maximum continuous dosage is 6 mg/hour administered over the waking day (e.g., 16 hours).
Extra Dose
Extra doses of ONAPGO may be used:
- upon starting in the morning; or
- when restarting the continuous dosage after a 1-hour or longer break in use (i.e., as a loading dose); or
- as a supplement to the continuous dosage to manage acute OFF symptoms that are not controlled
The extra dose may be titrated to clinical response and tolerability with adjustments in increments of 0.5 mg or 1 mg. Subsequent extra doses may be between 0.5 mg and 2 mg.
Administer no more than 3 extra doses per day over 16 hours with at least 3 hours between extra doses.
If 3 extra doses are routinely required during daily infusion, consider further adjustment of the continuous dosage.
The maximum recommended total daily dosage, including extra doses, is 98 mg during the waking day (e.g., 16 hours).
2.4 Preparation and Administration Instructions
Patients and caregivers should receive education and be trained on the proper use of ONAPGO and the device delivery system prior to first use [see Dosage and Administration (2.2)and Patient Instructions for Use].
- Visually inspect solution for particulate matter and discoloration prior to administration. Solution should be clear, almost colorless. Do not use if solution is discolored or contains particles.
- Keep the infusion site clean and use aseptic technique.
- Administer ONAPGO subcutaneously in one of the following areas:
- Abdomen at least 2 inches away from the navel
- Top of thigh
- Lower back
- Upper back, only when the infusion site is being prepared by a caregiver or healthcare provider
- Change the infusion site every day
- Do notselect an infusion site that is bruised, has bumps or nodules, or is irritated.
- A new single-dose cartridge and cartridge holder should be used each day. Discard unused portion.
2.5 Recommended Dosage in Patients with Renal Impairment
For patients with mild or moderate renal impairment, the recommended initial extra dose is 0.5 mg to 1 mg and should not exceed 1 mg [see Clinical Pharmacology (12.3)and Use in Specific Populations (8.6)]. No adjustment is needed for the continuous dose, maximum recommended daily dose, or subsequent extra doses after the initial extra dose [see Dosage and Administration (2.3)]. Patients with severe renal impairment have not been studied.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
ONAPGO is contraindicated in patients:
- Using concomitant 5HT 3antagonists, including antiemetics (e.g., ondansetron, granisetron, dolasetron, palonosetron) and alosetron [see Drug Interactions (7.1)] . There have been reports of profound hypotension and loss of consciousness when apomorphine was administered with ondansetron.
- With hypersensitivity/allergic reaction to apomorphine or any of the excipients of ONAPGO, including sulfite (i.e., sodium metabisulfite). Angioedema or anaphylaxis may occur [see Warnings and Precautions (5.13)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Adverse Reactions After Intravenous Administration
Following intravenous administration of apomorphine, serious adverse reactions including thrombus formation and pulmonary embolism caused by intravenous crystallization of apomorphine have occurred. Do not administer ONAPGO intravenously.
5.2 Nausea and Vomiting
ONAPGO is known to cause nausea and vomiting, which may be severe. In Study 1 [see Clinical Studies (14)] 87% of patients were pretreated with an antiemetic. Twenty-two percent of patients treated with ONAPGO reported nausea and 7% reported vomiting, compared to 9% and 4%, respectively, of patients who received placebo. The ability of concomitantly administered antiemetic drugs (other than trimethobenzamide) to reduce apomorphine-associated nausea/vomiting has not been studied. Antiemetics with anti-dopaminergic actions (e.g., haloperidol, chlorpromazine, promethazine, prochlorperazine, metoclopramide) have the potential to worsen the symptoms in patients with PD and therefore the benefits and risks of treatment with these antiemetics should be carefully considered. The use of ONAPGO with 5HT 3antiemetics is contraindicated.
5.3 Falling Asleep During Activities of Daily Living and Somnolence
Somnolence is commonly associated with ONAPGO .Patients treated with dopaminergic medications, including apomorphine, have also reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles, which sometimes resulted in accidents. Patients may not perceive warning signs, such as excessive drowsiness, or they may report feeling alert immediately prior to the event. In the double-blind placebo-controlled trial (Study 1), somnolence was reported in 22% of patients treated with ONAPGO and 4% of patients in the placebo group.
Falling asleep while engaged in activities of daily living usually occurs in patients experiencing preexisting somnolence, although patients may not give such a history. Therefore, prescribers should reassess patients for drowsiness or sleepiness while using ONAPGO, especially because some of the events occur well after the start of treatment. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities.
Before initiating treatment with ONAPGO, advise patients of the risk of drowsiness and ask them about factors that could increase the risk with ONAPGO, such as concomitant sedating medications and the presence of sleep disorders. If a patient develops significant daytime sleepiness or falls asleep during activities that require active participation (e.g., conversations, eating, etc.), ONAPGO should ordinarily be discontinued. If a decision is made to continue ONAPGO, patients should be advised not to drive and to avoid other potentially dangerous activities. There is insufficient information to determine whether dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
5.4 Syncope/Hypotension/Orthostatic Hypotension
Dopamine agonists, including ONAPGO, may cause orthostatic hypotension at any time, but especially during dose escalation. Patients with PD may also have an impaired capacity to respond to an orthostatic challenge. For these reasons, PD patients being treated with dopaminergic agonists should be monitored for signs and symptoms of orthostatic hypotension, especially during dose escalation, and should be informed of this risk.
When evaluated at various times after dosing in Study 1, patients undergoing titration of ONAPGO showed an increased incidence (from 4.5% pre-dose to 44.4% post-dose) of systolic orthostatic hypotension (≥ 20 mm Hg decrease upon standing) compared to placebo (7.1% pre-dose to 16.3% post-dose). Similarly, increased incidences of diastolic orthostatic hypotension (≥ 10 mm Hg decrease upon standing) were noted in patients titrated with ONAPGO (4.5% pre-dose to 44.4% post-dose) compared to placebo (11.9% pre-dose to 30.2% post-dose). Through the 12-week treatment period, these differences in incidence rates for systolic and diastolic orthostatic hypotension remained similar between ONAPGO and placebo patients. Patients in both ONAPGO and placebo groups developed severe systolic orthostatic hypotension (≥ 40 mm Hg decrease) and/or diastolic orthostatic hypotension (≥ 20 mm Hg decrease).
In Study 1, 13% of patients treated with ONAPGO reported hypotension or orthostatic hypotension compared to 2% of patients who received placebo [hypotension (7% vs 0%), orthostatic hypotension (4% vs 2%), and orthostatic intolerance (2% vs 0%)]. In the double-blind period of Study 1, one patient (2%) experienced a serious adverse event of hypotension and withdrew from the study compared to none in placebo.
Syncope has been reported in patients who have received subcutaneous apomorphine.
Patients with PD may also have an impaired capacity to respond to an orthostatic challenge. For these reasons, PD patients being treated with dopaminergic agonists ordinarily require careful monitoring for signs and symptoms of orthostatic hypotension, especially during dose escalation, and should be informed of this risk. Patients should avoid alcohol when using ONAPGO [see Drug Interactions (7.3)] . Patients taking ONAPGO should lie down before and after taking sublingual nitroglycerin. Other vasodilators and antihypertensives may also increase the hypotensive effects of ONAPGO. Monitor blood pressure for hypotension and orthostatic hypotension in patients taking ONAPGO with concomitant antihypertensive medications or vasodilators [see Drug Interactions (7.2)].
5.5 Falls
Patients with Parkinson's disease are at risk of falling due to underlying postural instability, possible autonomic instability, and syncope caused by the blood pressure lowering effects of the drugs used to treat Parkinson's disease. ONAPGO might increase the risk of falling by simultaneously lowering blood pressure and altering mobility [see Warnings and Precautions (5.4)and Clinical Pharmacology (12.2)]. Falls, including serious falls, have been reported in patients treated with apomorphine.
5.6 Infusion Site Reactions and Infections
ONAPGO can cause infusion site reactions and infections.
In Study 1, one or more infusion site reactions were reported in 63% of patients treated with ONAPGO and 15% in patients who received placebo. Various types of reactions at the infusion site have been reported including nodules, erythema, hematomas, inflammation, pruritus, swelling, discoloration, hemorrhage, hypersensitivity, induration, edema, pain, rash, or bruising [see Adverse Reactions (6.1)] . In Study 1, 2% of patients treated with ONAPGO and no patient who received placebo discontinued treatment because of an infusion site reaction.
Infusion site infections occurred in 4% of patients treated with ONAPGO compared to no patients who received placebo. The most frequent infusion site infection reported was cellulitis. If an infection is suspected at the infusion site, the cannula should be removed from the infusion site. If the cannula is removed for an infection, a new cannula should be placed at a new infusion site. In the event of a prolonged interruption of treatment with ONAPGO, the patient should be prescribed oral medications to treat their Parkinson's disease [see Dosage and Administration (2.4)].
5.7 Hallucinations/Psychotic-Like Behavior
In Study 1, 4% of patients treated with ONAPGO reported hallucinations compared to 4% of patients who received placebo. Psychotic disorder was reported in 2% of patients treated with ONAPGO compared to none who received placebo.
Postmarketing reports with intermittent subcutaneous apomorphine indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic-like behavior after starting or increasing the dose of apomorphine. Other drugs prescribed to improve the symptoms of PD can have similar effects on thinking and behavior. This abnormal thinking and behavior can consist of one or more of a variety of manifestations, including paranoid ideation, delusions, hallucinations, confusion, disorientation, aggressive behavior, agitation, and delirium. Consider the risks and benefits prior to initiating treatment with ONAPGO in patients with a major psychotic disorder because of the risk of exacerbating psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of PD and may decrease the effectiveness of ONAPGO [see Drug Interactions (7.3)] .
5.8 Dyskinesia
ONAPGO may cause dyskinesia or exacerbate pre-existing dyskinesia. In Study 1, dyskinesia or worsening of dyskinesia was reported in approximately 15% of patients treated with ONAPGO compared to no patients who received placebo.
5.9 Hemolytic Anemia
Hemolytic anemia requiring hospitalization has been reported with apomorphine treatment in a postmarketing setting. Many of the reported cases included positive direct antiglobulin test (Coombs test), suggesting a potential immune-mediated hemolysis. Severe anemia, angina, and dyspnea have occurred with hemolytic anemia. Some patients were treated with high dose glucocorticoids or blood transfusions. Hemolytic anemia can occur at any time after apomorphine treatment. If a patient develops anemia while taking ONAPGO, consider a workup for hemolytic anemia. If hemolytic anemia occurs, consider discontinuing treatment with ONAPGO. In Study 1, hemolytic anemia was reported in 2% of patients treated with ONAPGO compared to no patient who received placebo.
5.10 Impulse Control/Compulsive Behaviors
Patients may experience intense urges to gamble, increased sexual urges, intense urges to spend money uncontrollably, and other intense urges and the inability to control these urges while taking one or more of the medications, including ONAPGO, that increase central dopaminergic tone and that are generally used for the treatment of PD. In some cases, although not all, these urges were reported to have stopped when the dose was reduced, or the medication was discontinued. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending, or other urges while being treated with ONAPGO. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking ONAPGO
5.11 Cardiac Events
In Study 1, 4 patients treated with ONAPGO experienced cardiac disorders, including left bundle branch block (2%), myocardial infarction (2%), palpitations (2%), or tachycardia (2%), compared to no patients who received placebo. In patients treated with apomorphine subcutaneous injection, cardiac arrest and/or sudden death has occurred; some cases of angina and myocardial infarction occurred in close proximity to apomorphine dosing (within 2 hours), while other cases of cardiac arrest and sudden death were observed at times unrelated to dosing.
ONAPGO has been shown to reduce resting systolic and diastolic blood pressure and may have the potential to exacerbate cardiac (and cerebral) ischemia in patients with known cardiovascular and cerebrovascular disease. If patients develop signs and symptoms of cardiac or cerebral ischemia, prescribers should re-evaluate the continued use of ONAPGO.
5.12 QTc Prolongation and Potential for Proarrhythmic Effects
There is a dose-related prolongation of QTc interval after apomorphine exposure similar to that achieved with therapeutic doses of ONAPGO [see Clinical Pharmacology (12.2)] . In Study 1, 4% of patients treated with ONAPGO experienced prolonged QTc interval compared to none who received placebo.
Drugs that prolong the QTc interval have been associated with torsades de pointes and sudden death. The relationship of QTc prolongation to torsades de pointes is clearest for larger increases (20 msec and greater), but it is possible that smaller QTc prolongations may also increase risk, or increase it in susceptible individuals, such as those with hypokalemia, hypomagnesemia, bradycardia, concomitant use of other drugs that prolong the QTc interval, or genetic predisposition (e.g., congenital prolongation of the QT interval). Although torsades de pointes has not been observed in association with the use of ONAPGO at recommended doses in clinical studies, experience is too limited to rule out an increased risk. Palpitations and syncope may signal the occurrence of an episode of torsades de pointes.
The risks and benefits of ONAPGO treatment should be considered prior to initiating treatment with ONAPGO in patients with risk factors for prolonged QTc.
5.13 Hypersensitivity
In Study 1, 11% of patients treated with ONAPGO reported rash (4%), infusion site pruritus (4%), infusion site rash (2%), hypersensitivity (2%), or infusion site hypersensitivity (2%) compared to no patient who received placebo. Hypersensitivity/allergic reactions characterized by urticaria, rash, pruritus, and/or various manifestations of angioedema may occur because of ONAPGO or because of its sulfite excipient. ONAPGO contains sodium metabisulfite, a sulfite that may cause allergic-type reactions, including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
5.14 Fibrotic Complications
Cases of retroperitoneal fibrosis, pulmonary infiltrates, pleural effusion, pleural thickening, and cardiac valvulopathy have been reported in some patients treated with ergot-derived dopaminergic agents. While these complications may resolve when the drug is discontinued, complete resolution does not always occur. Although these adverse reactions are believed to be related to the ergoline structure of these dopamine agonists, whether other, non-ergot-derived dopamine agonists, such as ONAPGO, can cause these reactions is unknown.
5.15 Priapism
In clinical studies of intermittent apomorphine injection, painful erections were reported in 3 of 361 men treated with subcutaneous injection, and one patient withdrew from therapy because of priapism. Although no patients in the clinical studies required surgical intervention, severe priapism may require surgical intervention.
5.16 Retinal Pathology in Albino Rats
In a two-year carcinogenicity study of apomorphine in albino rat, retinal atrophy was detected at all subcutaneous doses tested (up to 0.8 mg/kg/day or 2 mg/kg/day in males or females, respectively; less than the maximum recommended human dose (MRHD) of 98 mg/day on a body surface area [mg/m 2] basis). Retinal atrophy/degeneration has been observed in albino rats treated with other dopamine agonists for prolonged periods (generally during two-year carcinogenicity studies). Retinal findings were not observed in a 39-week subcutaneous toxicity study of apomorphine in monkey at doses up to 1.5 mg/kg/day, a dose similar to the MRHD on a mg/m 2basis. The clinical significance of the finding in rat has not been established but cannot be disregarded because disruption of a mechanism that is universally present in vertebrates (e.g., disk shedding) may be involved.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Serious Adverse Reactions After Intravenous Administration [see Warnings and Precautions (5.1)]
- Nausea and Vomiting [see Warnings and Precautions (5.2)]
- Falling Asleep During Activities of Daily Living and Somnolence [see Warnings and Precautions (5.3)]
- Syncope/Hypotension/Orthostatic Hypotension [see Warnings and Precautions (5.4)]
- Falls [see Warnings and Precautions (5.5)]
- Infusion Site Reactions and Infections [see Warnings and Precautions (5.6)]
- Hallucinations/Psychotic-Like Behavior [see Warnings and Precautions (5.7)]
- Dyskinesia [see Warnings and Precautions (5.8)]
- Hemolytic Anemia [see Warnings and Precautions (5.9)]
- Impulse Control/Compulsive Behaviors [see Warnings and Precautions (5.10)]
- Cardiac Events [see Warnings and Precautions (5.11)]
- QTc Prolongation and Potential for Proarrhythmic Effects [see Warnings and Precautions (5.12)]
- Hypersensitivity [see Warnings and Precautions (5.13)]
- Fibrotic Complications [see Warnings and Precautions (5.14)]
- Priapism [see Warnings and Precautions (5.15)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. A total of 197 Parkinson's disease patients were exposed to ONAPGO in Study 1 and subsequent open-label studies.
In Study 1 [see Clinical Studies (14)], the most common adverse reactions (incidence 10% or greater in patients treated with ONAPGO and at a rate at least twice the placebo rate) were infusion site nodule, nausea, somnolence, infusion site erythema, dyskinesia, headache, and insomnia.
Table 1 presents adverse reactions that occurred in ≥5% of patients treated with ONAPGO and that occurred more frequently than placebo in patients who were enrolled in Study 1.
Table 1: Adverse Reactions Reported by ≥5% of Patients Treated with ONAPGO and More Frequently than Placebo in Study 1 ONAPGO *
N=54
%Placebo
N=53
%- * Variable doses [see Dosage and Administration (2.3)]
Infusion site nodule 44 0 Nausea 22 9 Somnolence 22 4 Infusion site erythema 17 4 Dyskinesia 15 0 Headache 13 4 Insomnia 11 2 Dizziness 9 4 Hypotension 7 0 Asthenia 7 0 Fatigue 7 2 Constipation 7 6 Vomiting 7 4 Infusion Site Reactions
In Study 1, infusion site reactions were most commonly reported as nodule formation (44% of patients treated with ONAPGO compared to no patients who received placebo) or erythema (17% of patients treated with ONAPGO compared to 4% of patients who received placebo).
Less Common Adverse Reactions
Other adverse reactions, including hallucinations, psychotic disorder, impulse control disorder, prolonged QT interval, and infusion site infections [see Warnings and Precautions (5.6)] , have been reported in patients treated with ONAPGO [see Warnings and Precautions (5.7, 5.10. 5.12)].
Adverse Reactions Leading to Discontinuation of ONAPGO Treatment
Six (11%) of patients treated with ONAPGO reported one of the following adverse reactions as the reason for discontinuing from Study 1: nausea, orthostatic intolerance, visual hallucination, gait disturbance, infusion site nodule, and hypotension. None of the patients who received placebo discontinued from Study 1 because of an adverse reaction.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of apomorphine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hematologic and Lymphatic Systems: hemolytic anemia [see Warnings and Precautions (5.9)]
-
7 DRUG INTERACTIONS
7.1 5HT 3Antagonists
Based on reports of profound hypotension and loss of consciousness when apomorphine was administered with ondansetron, the concomitant use of ONAPGO with 5HT 3antagonists, including antiemetics (e.g., ondansetron, granisetron, palonosetron) and alosetron, is contraindicated [see Warnings and Precautions (5.4)].
7.2 Antihypertensive Medications and Vasodilators
In clinical studies with intermittent subcutaneous administration of apomorphine, the following adverse reactions were experienced more commonly in patients receiving concomitant antihypertensive medications or vasodilators (n=94) than in patients not receiving these medications (n=456): hypotension (10% vs 4%) myocardial infarction (3% vs 1%), [see Warnings and Precautions (5.4, 5.11)].
In a study of healthy subjects, concomitant administration of 0.4 mg sublingual nitroglycerin with subcutaneous apomorphine caused greater decreases in blood pressure than with subcutaneous apomorphine alone [see Clinical Pharmacology (12.3)]. Patients treated with ONAPGO should lie down before and after taking sublingual nitroglycerin to decrease the potential risks associated with hypotension .
7.3 Alcohol
In a study of healthy subjects, concomitant administration of high dose (0.6 g/kg) or low dose (0.3 g/kg) ethanol with apomorphine caused greater decreases in blood pressure than with apomorphine alone [see Clinical Pharmacology (12.3)] . Patients should be advised not to consume alcohol while treated with ONAPGO because it may cause greater decreases in blood pressure [see Warnings and Precautions (5.4)] .
7.4 Dopamine Antagonists
Because ONAPGO is a dopamine agonist, it is possible that concomitant use of dopamine antagonists, such as the neuroleptics (phenothiazines, butyrophenones, thioxanthenes) or metoclopramide, may diminish the effectiveness of ONAPGO. Antiemetics with anti-dopaminergic actions should be avoided [see Warnings and Precautions (5.2)]. Patients with major psychotic disorders, treated with neuroleptics, should be treated with dopamine agonists only if the potential benefits outweigh the risks [see Warnings and Precautions (5.7)].
7.5 Drugs Prolonging the QT/QTc Interval
Caution should be exercised when prescribing ONAPGO concomitantly with drugs that prolong the QT/QTc interval [see Warnings and Precautions (5.12)and Clinical Pharmacology (12.2)] .
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with use of ONAPGO in pregnant women. In animal reproduction studies, apomorphine had adverse developmental effects in rats (increased neonatal deaths) and rabbits (increased incidence of malformation) when administered during pregnancy at clinically relevant doses. These doses were also associated with maternal toxicity. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal Data
No adverse developmental effects were observed when apomorphine (0.3, 1, or 3 mg/kg/day) was administered by subcutaneous injection to pregnant rats throughout organogenesis; the highest dose tested is less than the maximum recommended human dose (MRRD) of 98 mg on a mg/m 2basis. Administration of apomorphine (0.3, 1, or 3 mg/kg/day) by subcutaneous injection to pregnant rabbits throughout organogenesis resulted in an increased incidence of malformations of the heart and/or great vessels at the mid and high doses; maternal toxicity was observed at the highest dose tested. The no-effect dose (0.3mg/kg/day) for adverse effects is less than the MRHD on a mg/m 2basis.
Apomorphine (0.3, 1, or 3 mg/kg/day), administered by subcutaneous injection to female rats throughout gestation and lactation, resulted in increased offspring mortality at the highest dose tested, which was associated with maternal toxicity. There were no effects on developmental parameters or reproductive performance in surviving offspring. The no-effect dose (1 mg/kg/day) for developmental toxicity is less than the MRHD on a mg/m 2basis.
8.2 Lactation
Risk Summary
There are no data on the presence of apomorphine in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ONAPGO and any potential adverse effects on the breastfed infant from ONAPGO or from the underlying maternal condition.
8.5 Geriatric Use
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of comorbid disease or other drug therapy.
Clinical studies of ONAPGO did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger subjects. Clinical experience with intermittent subcutaneous use of apomorphine has shown that the following adverse reactions were reported more frequently in patients 65 years of age or older (n=311) compared to patients less than 65 years of age (n=239): confusion; hallucinations; serious adverse reactions (life-threatening events or events resulting in hospitalization and/or increased disability); falls (experiencing bone and joint injuries); cardiovascular events; respiratory disorders; gastrointestinal events; and discontinuation of treatment as a result of one or more adverse reactions.
8.6 Renal Impairment
The initial extra dose of ONAPGO should not exceed 1 mg in patients with mild or moderate renal impairment because of increased apomorphine concentration and exposure (C maxand AUC) in these patients. Studies in patients with severe renal impairment have not been conducted [see Dosage and Administration (2.5)and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Exercise caution and closely monitor if ONAPGO is administered to patients with mild or moderate hepatic impairment because of increased apomorphine concentration and exposure (C maxand AUC) in these patients. Studies in patients with severe hepatic impairment have not been conducted [see Clinical Pharmacology (12.3)] .
-
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
In premarketing clinical experience, apomorphine did not reveal any tendency for a withdrawal syndrome or any drug-seeking behavior. However, there are rare postmarketing reports of abuse of medications containing apomorphine. In general, these reports consist of patients taking increasing doses of medication in order to achieve a euphoric state. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects.
-
11 DESCRIPTION
ONAPGO (apomorphine hydrochloride) contains apomorphine hydrochloride hemihydrate, a non-ergoline dopamine agonist. It is chemically designated as 6aβ-aporphine-10,11-diol hydrochloride hemihydrate with a molecular formula of C 17H 17NO 2∙HCl∙½H 2O. Its structural formula and molecular weight are:
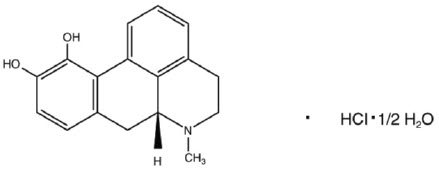
Apomorphine hydrochloride hemihydrate appears as minute, white or grayish-white glistening crystals or as white powder that is soluble in water at 80°C. ONAPGO injection is a clear, almost colorless, sterile solution for subcutaneous infusion and is available in 98 mg/20 mL (4.9 mg/mL) single-dose prefilled cartridges. The solution has a pH of 3.0 -4.0. Each mL contains 4.9 mg of apomorphine hydrochloride (equivalent to 4.27 mg apomorphine) and 0.5 mg of sodium metabisulfite in water for injection. In addition, each mL may contain hydrochloric acid used to adjust the pH.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
ONAPGO is a non-ergoline dopamine agonist with high in vitro binding affinity for the dopamine D4 receptor, and moderate affinity for the dopamine D2, D3, and D5, and adrenergic α1D, α2B, α2C receptors. The precise mechanism of action of ONAPGO as a treatment for Parkinson's disease is unknown, although it is believed to be due to stimulation of post-synaptic dopamine D2-type receptors within the caudate-putamen in the brain.
12.2 Pharmacodynamics
Prolongation of the QTc Interval and Cardiac Electrophysiology
In a thorough QT study, the mean increase in QTcF was 12.5 ms (90% two-sided upper confidence interval = 18.0 ms) after administration of a continuous dose of ONAPGO 6 mg/hr (maximum recommended infusion rate) in healthy subjects. A mean increase in QTcF of 14.6 ms (90% two-sided confidence interval = 20.1 ms) was observed at a dose and exposure similar to that expected with the maximum recommended total daily dosage of 98 mg. The maximum placebo-corrected increase in heart rate was 14.1 bpm (90% two-sided upper confidence interval = 17.4 bpm) at the dose and exposure similar to that expected with the maximum recommended total daily dose of 98 mg.
12.3 Pharmacokinetics
Absorption
After subcutaneous administration, apomorphine appears to have bioavailability equal to that of intravenous administration. Steady-state concentrations are reached in approximately two hours after initiation of a continuous dose. If an extra dose is administered at the initiation of the continuous dose [see Dosage and Administration (2.3)] , time to reach half (50%) of infusion steady-state concentrations can be shortened by 50-75%. If an extra dose is administered two hours or later following initiation of a continuous dose, the supplemental exposure from the extra dose reaches peak concentration in 20-40 minutes.
Distribution and Binding
The plasma-to-whole blood apomorphine concentration ratio is equal to one. Mean (range) apparent volume of distribution was 218 L (123 to 404 L). Protein binding in humans is 89% and concentration independent.
Elimination
The mean apparent clearance of apomorphine from plasma is 261 to 280 L/hr for ONAPGO with infusion rates ranging from 2 to 6 mg/hr. After administration of an extra dose, the mean elimination half-life is approximately 40 minutes.
Metabolism
Apomorphine metabolism is primarily via conjugation. Within 0.5 hours of administering a subcutaneous dose of 14C-apomorphine, only 8.3% of plasma radioactivity was apomorphine, while 83% was associated with the apomorphine sulfate, 3.5% with apomorphine glucuronide, and 1% with nor-apomorphine glucuronide.
Excretion
Eighty-six percent of the subcutaneously administered dose of 14C-apomorphine was recovered in the urine within 48 hours as apomorphine sulfate, apomorphine glucuronide and nor-apomorphine glucuronide. Another 4.6% of the administered dose was recovered in the feces over 144 hours as apomorphine sulfate, apomorphine glucuronide, and apomorphine.
Specific Populations
No clinically significant differences in the pharmacokinetics of apomorphine were observed based on age, gender, weight, duration of Parkinson's disease, levodopa dose, or duration of therapy.
Renal Impairment
In a study comparing patients with renal impairment (moderately impaired as determined by estimated creatinine clearance) to healthy matched volunteers, the AUC 0-infand C maxwere increased by approximately 16% and 50%, respectively, following a single subcutaneous administration of apomorphine into the abdominal wall. The mean time to peak concentrations and the mean terminal half-life of apomorphine were unaffected by the renal status of the individual. Studies in patients with severe renal impairment have not been conducted [see Dosage and Administration (2.5)and Use in Specific Populations (8.6)] .
Hepatic Impairment
In a study comparing patients with hepatic impairment (moderately impaired as determined by the Child-Pugh classification method) to healthy matched volunteers, the AUC 0-infand C maxvalues were increased by approximately 10% and 25%, respectively, following a single subcutaneous administration of apomorphine into the abdominal wall. Studies in patients with severe hepatic impairment have not been conducted [see Use in Specific Populations (8.7)] .
Drug Interaction Studies
Carbidopa/levodopa
Pharmacokinetics of levodopa were unchanged when subcutaneous apomorphine and levodopa were co-administered in patients. However, motor response differences were significant.
Ethanol and Nitroglycerin
Co-administration of low dose ethanol (0.3 g/kg), or nitroglycerin (0.4 mg) with an apomorphine injection dose of 6 mg in healthy individuals did not have a significant impact on the pharmacokinetics of apomorphine, but high dose ethanol (0.6 g/kg), equivalent to approximately 3 standardized alcohol-containing beverages, increased the C maxof apomorphine by about 63%. The hypotensive effect of apomorphine was increased by the concomitant use of alcohol or of sublingual nitroglycerin. When high-dose ethanol (0.6 g/kg) and subcutaneous apomorphine were concomitantly administered to healthy subjects, the mean largest decrease (the mean of each subject's largest drop in blood pressure measured within the 6-hour period following administration of subcutaneous apomorphine) for supine systolic and diastolic blood pressure was 9.1 mm Hg and 10.5 mm Hg, respectively. The mean largest standing systolic and diastolic blood pressure decrease was 11.3 mm Hg and 12.6 mm Hg, respectively. In some individuals, the decrease was as high as 61 mm Hg and 51 mm Hg, respectively, for standing systolic and diastolic blood pressure [see Warnings and Precautions (5.4), Drug Interactions (7.2, 7.3)].
Other Drugs Eliminated Via Hepatic Metabolism
Based upon an in vitrostudy, cytochrome P450 enzymes play a minor role in the metabolism of apomorphine. In vitrostudies have also demonstrated that drug interactions are unlikely due to apomorphine acting as a substrate, an inhibitor, or an inducer of cytochrome P450 enzymes.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Lifetime carcinogenicity studies of apomorphine were conducted in male (0.1, 0.3, or 0.8 mg/kg/day) and female (0.3, 0.8, or 2 mg/kg/day) rats. Apomorphine was administered by subcutaneous injection for 22 months or 23 months, respectively. In males, there was an increase in Leydig cell tumors at the highest dose tested. This finding is of questionable significance because the endocrine mechanisms believed to be involved in the production of Leydig cell tumors in rats are not relevant to humans. No drug-related tumors were observed in females. The highest doses tested in males and females are less than the maximum recommended human dose (MRHD) on a mg/m 2basis.
In a 26-week carcinogenicity study in p53-knockout transgenic mice, there was no evidence of carcinogenic potential when apomorphine was administered by subcutaneous injection at doses up to 20 mg/kg/day in males or 40 mg/kg/day in females.
Mutagenesis
Apomorphine was mutagenic in the in vitrobacterial reverse mutation (Ames) and the in vitromouse lymphoma tkassays. Apomorphine was clastogenic in the in vitrochromosomal aberration assay in human lymphocytes and in the in vitromouse lymphoma tkassay. Apomorphine was negative in the in vivomicronucleus assay in mice.
Impairment of Fertility
Apomorphine was administered subcutaneously at doses up to 3 mg/kg/day to male and female rats prior to and throughout the mating period and continuing in females through gestation day 6. There was no evidence of adverse effects on fertility or on early fetal viability. The highest dose tested is less than the MRHD on a mg/m 2basis. A significant decrease in testis weight was observed in a 39-week study in cynomolgus monkey at all subcutaneous doses tested (0.3, 1, or 1.5 mg/kg/day); the lowest dose tested is less than the MRHD on a mg/m 2basis.
In a published fertility study, apomorphine was administered to male rats at subcutaneous doses of 0.2, 0.8, or 2 mg/kg prior to and throughout the mating period. Fertility was reduced at the highest dose tested.
-
14 CLINICAL STUDIES
Study 1 (NCT02006121) was a multicenter, parallel-group, double-blind, randomized, placebo-controlled trial to evaluate the efficacy and safety of ONAPGO subcutaneous infusion in patients with Parkinson's disease who had motor fluctuations while receiving carbidopa/levodopa and other concomitant medications to treat PD. The study design included a double-blind, 12-week treatment period, inclusive of 1-4 weeks of dose titration and then continued treatment with study medication at an individualized, stable dosage. Patients then had the option to receive ONAPGO in a 52-week, open-label treatment period.
The modified intent-to-treat (mITT) population included 53 patients treated with ONAPGO and 51 patients who received placebo. At baseline, patients experienced OFF episodes averaging ≥3 hours per day. In the mITT population, the mean age was 63.4 years, 62% were male, and 100% were White. At baseline, all patients were taking at least one concomitant medication to treat PD (e.g., dopamine agonist 87%, COMT-inhibitor 58%, MAO-B inhibitor 40%, amantadine 27%) in addition to oral immediate or extended-release levodopa plus a dopa decarboxylase inhibitor. The mean duration of PD was 11.9 years in the ONAPGO-treated group and 10.8 years in the placebo-treated group.
During titration, adjustments in concomitant medications to treat PD were made when clinically indicated. All patients treated with ONAPGO in the double-blind study were maintained on levodopa.
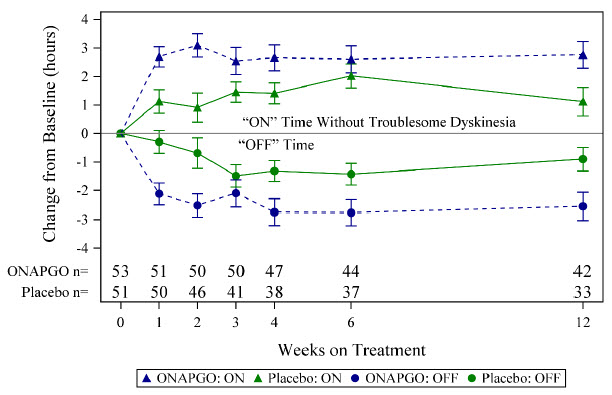
The primary efficacy endpoint for Study 1 was the change in total daily OFF time assessed from baseline to the end of the 12-week treatment period based on patient diaries. A key secondary endpoint was the change in daily ON time without troublesome dyskinesia from baseline to the end of the 12-week treatment period. There was a statistically significant reduction in the amount of daily OFF time in patients treated with ONAPGO compared to placebo (p=0.0114; see Table 2). There was also a statistically significant increase in daily ON time without troublesome dyskinesia in patients treated with ONAPGO compared to placebo (p=0.0188; see Table 3).
Table 2: Change From Baseline to Week 12 in Mean Total Daily OFF Time in Study 1 (mITT population) N Baseline (hours)
(mean ± SD)LS Mean Change from Baseline at Week 12
(hours)
(mean ± SE)LSD *vs Placebo
(95% CI) †
p-value- * Least squares difference from placebo; a negative value indicates improvement (reduction in OFF time)
- † 95% CI: 95% Confidence Interval
Placebo 51 6.72 ± 2.547 -0.90 ± 0.416 -- ONAPGO 53 6.69 ± 2.224 -2.55 ± 0.487 -1.65
(-2.91, -0.38)
p=0.0114Table 3: Change From Baseline to Week 12 in Mean Daily ON Time Without Troublesome Dyskinesia in Study 1 (mITT population) N Baseline (hours)
(mean ± SD)LS Mean Change from Baseline at Week 12
(hours)
(mean ± SE)LSD *vs Placebo
(95% CI) †
p-value- * Least squares difference from placebo; a positive value indicates improvement (increase in ON time without troublesome dyskinesia)
- † 95% CI: 95% Confidence Interval
Placebo 51 8.64 ± 2.496 1.12 ± 0.500 -- ONAPGO 53 8.56 ± 2.329 2.76 ± 0.471 1.64
(0.28, 3.00)
p=0.0188In addition, the time course showed numerically greater improvement in daily OFF time and daily ON time without troublesome dyskinesia in patients treated with ONAPGO compared to placebo, at all post-baseline timepoints (Figure 2).
Figure 2: Mean Change from Baseline in Daily OFF Time and ON Time Without Troublesome Dyskinesia
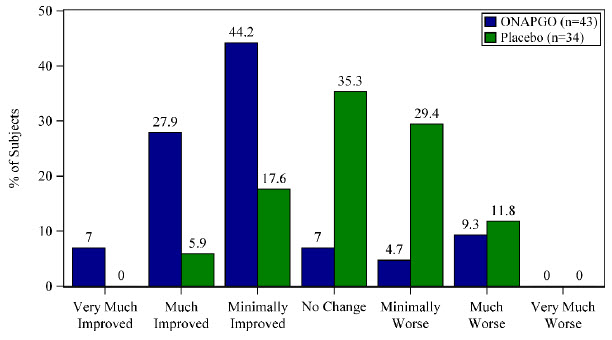
A patient reported outcome, the Patient Global Impression of Change (PGIC) was also assessed. The PGIC evaluates the change in Parkinson's disease symptoms as perceived by the patient in a seven-point single-item scale ranging from 'very much worse' to 'very much improved' (Figure 3).
Figure 3: Week 12 Patient Global Impression of Change
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
ONAPGO injection is supplied as one carton (NDC: 27505-006-05) that contains five single-dose prefilled glass cartridges, each containing 98 mg/20 mL (4.9 mg/mL) apomorphine hydrochloride as a clear, almost colorless solution.
The ONAPGO Pump Kit, ONAPGO Cartridge Holders, and appropriate infusion sets are supplied separately.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Administration Information
Refer patients to the Instructions for Use for complete administration instructions. Inform patients of aseptic technique and of subcutaneous administration site selection and rotation [see Dosage and Administration (2.4)].
Advise patients that a new single-dose cartridge and cartridge holder and a new infusion line should be used each day.
Advise patients that ONAPGO is intended only for subcutaneous use and must not be given intravenously because of the risk of serious complications, such as thrombus formation and pulmonary embolism due to crystallization [see Warnings and Precautions (5.1)] .
Hypersensitivity/Allergic Reactions
Advise patients that hypersensitivity/allergic reactions characterized by urticaria, rash, pruritus, and/or various manifestations of angioedema may occur because of ONAPGO or any of its excipients, including a sulfite (i.e., sodium metabisulfite). Inform patients with a sulfite sensitivity that they may experience various allergic-type reactions, including anaphylactic symptoms and life-threatening asthmatic attacks [see Warnings and Precautions (5.13)] . Advise patients who experience any hypersensitivity/allergic reaction to ONAPGO that they should not take ONAPGO again [see Contraindications (4)].
Nausea and Vomiting
Advise patients that they may experience nausea and vomiting, which may be severe. Advise patients that their ONAPGO dosage may be adjusted to start at 1mg/hr, and titration can be based upon effectiveness and tolerability. Treatment with an antiemetic that is a non-5HT3 antagonist may be used if needed. Oral trimethobenzamide 300 mg 3 times per day for 3 days prior to starting ONAPGO infusion has been used in clinical studies to help lessen these symptoms. Advise patients that ONAPGO taken with trimethobenzamide may increase the risk for somnolence, dizziness, and falls, and to consult their healthcare provider before discontinuing trimethobenzamide [see Warnings and Precautions (5.2)].
Falling Asleep Suddenly and Sedation/Sleepiness
Alert patients to the potential sedating effects of ONAPGO, including somnolence and falling asleep while engaged in activities of daily living. Instruct patients not to drive a car or engage in other potentially dangerous activities until they have gained sufficient experience with ONAPGO to gauge whether or not it affects their mental and/or motor performance adversely. Advise patients that if increased somnolence or episodes of falling asleep during activities of daily living (e.g., watching television, passenger in a car, etc.) occur, they should not drive or participate in potentially dangerous activities until they have contacted their physician [see Warnings and Precautions (5.3)].
Hypotension/Orthostatic Hypotension
Advise patients that they may develop postural (orthostatic) hypotension with or without symptoms such as dizziness, nausea, syncope, and sometimes sweating. Hypotension and/or orthostatic symptoms may occur more frequently during initial therapy or with an increase in dose at any time (cases have been seen after months of treatment). Instruct patients to rise slowly after sitting or lying down while using ONAPGO. Inform patients that alcohol and nitroglycerin (and possibly other vasodilators and antihypertensive medications) may worsen the potential effects of hypotension caused by ONAPGO [see Warnings and Precautions (5.4)]. Instruct patients ideally to lie down before taking sublingual nitroglycerin and to remain supine and avoid standing for at least 45 minutes after nitroglycerin. Instruct patients to avoid alcohol while using ONAPGO.
Falls
Alert patients that they may have increased risk for falling when using ONAPGO [see Warnings and Precautions (5.5)].
Infusion Site Reactions and Infections
Inform patients that infusion of ONAPGO may result in infusion site reactions, including, but not limited to nodules, erythema, bruising, and infection. Infusion site infections were reported in <5% of patients on ONAPGO in clinical trials [see Warnings and Precautions (5.6)and Adverse Reactions (6.1)] . Instruct patients to change the infusion site every day and to observe proper aseptic technique [see Dosage and Administration (2.4)and Instructions for Use] .
Hallucinations and/or Psychotic-Like Behavior
Inform patients that ONAPGO may cause hallucinations or other manifestations of psychotic-like behavior. Advise patients to inform their healthcare provider if they have a major psychotic disorder or are taking any treatments for psychosis. Patients with a major psychotic disorder should also be aware that many treatments for psychosis may decrease the effectiveness of ONAPGO [see Warnings and Precautions (5.7)] .
Dyskinesia
Inform patients that ONAPGO may cause and/or exacerbate pre-existing dyskinesia [see Warnings and Precautions (5.8)].
Hemolytic Anemia
Inform patients and caregivers that hemolytic anemia may occur and to contact their healthcare provider if they develop any signs or symptoms [see Warnings and Precautions (5.9)] .
Impulse Control / Compulsive Behaviors
Patients and their caregivers should be alerted to the possibility that they may experience intense urges to spend money uncontrollably, intense urges to gamble, increased sexual urges, binge eating and/or other intense urges and the inability to control these urges while using ONAPGO [see Warnings and Precautions (5.10)].
Cardiac Events
Inform patients that ONAPGO may cause cardiac (and cerebral) ischemia, and these outcomes could possibly be related to significant hypotension/orthostatic hypotension [see Warnings and Precautions (5.11)].
QTc Prolongation and Potential for Proarrhythmic Effects
Alert patients that ONAPGO may cause QTc prolongation and might produce proarrhythmic effects that could cause torsades de pointes and sudden death. Palpitations and syncope may signal the occurrence of an episode of torsades de pointes [see Warnings and Precautions (5.12)].
Priapism
Advise patients that ONAPGO may cause prolonged painful erections and that if this occurs, they should seek medical attention immediately [see Warnings and Precautions (5.15)]
-
SPL UNCLASSIFIED SECTION
ONAPGO Manufactured for: MDD US Operations, LLC, 9715 Key West Ave., Rockville, MD 20850.
MDD US Operations, LLC is a subsidiary of Supernus Pharmaceuticals, Inc.
ONAPGO is a trademark of BRITUSWIP LTD.
All trademarks are the property of their respective owners.
© 2025 MDD US Operations, LLC, a subsidiary of Supernus Pharmaceuticals Inc.
RA-ONG-V1
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration Issued: 2/2025 PATIENT INFORMATION
ONAPGO™ (on-AP-goh)
(apomorphine hydrochloride)
injection, for subcutaneous useWhat is ONAPGO? ONAPGO is a prescription medicine used to treat motor fluctuations (OFF episodes) in adults with advanced Parkinson's disease. It is not known if ONAPGOis safe and effective in children. Who should not use ONAPGO? Do not use ONAPGO if you are: - taking certain medicines called 5HT3 antagonists, including medicines to treat nausea (ondansetron, granisetron, dolasetron, palonosetron) and alosetron. People taking ondansetron together with apomorphine, the active ingredient in ONAPGO, have had very low blood pressure and lost consciousness (blacked out).
- allergic to apomorphine or to any of the ingredients in ONAPGO including sulfite. See the end of this Patient Information leaflet for a complete list of ingredients in ONAPGO. Sulfites can cause severe, life-threatening allergic reactions in some people. An allergy to sulfites is not the same as an allergy to sulfa. People with asthma are also more likely to be allergic to sulfites.
Call your healthcare provider or get emergency help right away if you get any of the following symptoms of a severe life-threatening allergic reaction:
- hives
- itching
- rash
- swelling of the eyes, tongue, lips, or mouth
- chest pain
- throat tightness
- trouble breathing or swallowing
What should I tell my healthcare provider before using ONAPGO? Before you start using ONAPGO, tell your healthcare provider about all of your medical conditions, including if you: - have difficulty staying awake during the daytime.
- have dizziness.
- have fainting spells.
- have low blood pressure.
- have asthma.
- are allergic to any medicines containing sulfites.
- have heart problems.
- have had a stroke or other brain problems.
- have kidney problems.
- have liver problems.
- have a mental problem called a major psychotic disorder.
- drink alcohol.
- are pregnant or plan to become pregnant. It is not known if ONAPGO will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if ONAPGO passes into your breast milk. You and your healthcare provider should decide if you will take ONAPGO or breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription (over-the-counter) medicines, vitamins, and herbal supplements. ONAPGO and certain other medicines may affect each other and how they work. Using ONAPGO with other medicines can cause serious side effects. - If you take nitroglycerin under your tongue (sublingual) while using ONAPGO, your blood pressure may decrease and cause dizziness. If possible, you should lie down before taking nitroglycerin under your tongue, and then try to continue lying down for at least 45 minutes after you take nitroglycerin.
Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine. How should I use ONAPGO? - Read the Instructions for Use for specific information about the right way to use and to throw away (dispose of) ONAPGO.
- Use ONAPGO exactly as your healthcare provider tells you to use it.
- You will be taught the right way to use ONAPGO by your healthcare provider.
- Your healthcare provider will prescribe your dose of ONAPGO, and only they may change this dose if needed.
- Your healthcare provider or a nurse provided by the drug company will program your pump.
- Your healthcare provider will prescribe ONAPGO that comes in single-dose prefilled glass cartridges, that are used with the ONAPGO pump.
- Infuse ONAPGO under your skin (subcutaneously) only. Do not inject ONAPGO into a vein.
- Keep the infusion site clean, and change the infusion site every day.
- Use a new infusion set with each infusion. Never reuse an infusion set.
- ONAPGO is a clear and almost colorless liquid. Do not use ONAPGO if it looks cloudy, discolored, or contains particles.
- Your healthcare provider may prescribe trimethobenzamide to take while you are using ONAPGO, which is an antiemetic medicine to help decrease the symptoms of nausea and vomiting that can happen with ONAPGO.
What should I avoid while using ONAPGO? - Do notdrink alcohol while you are using ONAPGO. It can increase your chance of developing serious side effects.
- Do nottake medicines that make you sleepy while you are using ONAPGO.
- Do notdrive, operate machinery, or do other dangerous activities until you know how ONAPGO affects you.
- Do notchange your body position too fast. Get up slowly from sitting or lying. ONAPGO can lower your blood pressure and cause dizziness or fainting.
What are the possible side effects of ONAPGO? ONAPGO may cause serious side effects, including: - blood clots.Infusing ONAPGO into a vein (intravenous) can cause blood clots. Do notinfuse ONAPGO in your vein.
- nausea and vomiting are common side effects.Nausea and vomiting, which may be serious or severe, can happen with ONAPGO. Your healthcare provider may prescribe medicine (trimethobenzamide) to help decrease nausea and vomiting. Follow your healthcare providers instructions on how to take and when to stop this medicine.
- sleepiness or falling asleep during the day is a common side effect and may be serious.Some people treated with ONAPGO may get sleepy during the day or fall asleep without warning while doing everyday activities such as talking, eating, or driving a car.
- dizziness is a common side effect and may be serious.ONAPGO can lower your blood pressure and cause dizziness. Dizziness can happen when ONAPGO treatment is started or when the ONAPGO dose is increased. Do notget up too fast from sitting or after lying down, especially if you have been sitting or lying down for a long period of time.
- falls.The changes that can happen with PD, and the effects of some PD medicines as well as the effects of trimethobenzamide, can increase the risk of falling. ONAPGO may also increase your risk of falling.
- infusion site reaction is a common side effect that may be serious.Infusion site reactions and infections including infusion site nodules, redness, bruising, swelling, rash, and itching may happen during treatment with ONAPGO.
- hallucinations or psychotic-like behavior.ONAPGO can cause or worsen psychotic-like behavior including hallucinations (seeing or hearing things that are not real), confusion, excessive suspicion, aggressive behavior, agitation, delusional beliefs (believing things that are not real), and disorganized thinking.
- sudden uncontrolled movements (dyskinesia) are a common side effect and may be serious.Some people with PD may get sudden, uncontrolled movements after treatment with some PD medicines. ONAPGO can cause or make dyskinesia worse.
- low red blood cells (hemolytic anemia).Tell your healthcare provider if you have any of the following signs or symptoms: you become pale, fast heartbeat, feel more tired or weaker than usual, skin or eyes look yellow, chest pain, shortness of breath or trouble breathing, dark-colored urine, fever, dizziness, or confusion.
- strong (intense) urges.Some people with PD have reported new or increased gambling urges, increased sexual urges, and other intense urges, while taking PD medicines, including ONAPGO.
- heart problems.If you have shortness of breath, fast heartbeat, or chest pain while taking ONAPGO, call your healthcare provider or get emergency help right away.
- serious heart rhythm changes (QT prolongation).Tell your healthcare provider right away if you have a change in your heartbeat (a fast or irregular heartbeat), or if you faint.
- allergic reaction.An allergic reaction can happen when using ONAPGO. Tell your healthcare provider or get medical help right away if you get any of these symptoms: hives, itching, rash, swelling of the eyes and tongue, or trouble breathing.
- tissue changes (fibrotic complications).Some people have had changes in the tissues of their pelvis, lungs, and heart valves when taking medicines called non-ergot derived dopamine agonists like ONAPGO.
- prolonged painful erections (priapism). ONAPGO may cause prolonged, painful erections in some people. If you have an erection that lasts more than 4 hours you should call your healthcare provider or go to the nearest hospital emergency room right away.
Other common side effects of ONAPGO include: - headache
- trouble falling asleep or staying sleep (insomnia)
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of ONAPGO. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store ONAPGO? - Store ONAPGO at room temperature between 68°F to 77°F (20°C to 25°C).
Keep ONAPGO and all medicines out of the reach of children. General information about the safe and effective use of ONAPGO. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ONAPGO for a condition for which it was not prescribed. Do not give ONAPGO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about ONAPGO that is written for health professionals. What are the ingredients in ONAPGO? Active ingredient:apomorphine hydrochloride Inactive ingredients:hydrochloric acid, sodium metabisulfite in water for injection. Manufactured for: MDD US Operations, LLC, 9715 Key West Ave., Rockville, MD 20850. MDD US Operations, LLC is a subsidiary of Supernus Pharmaceuticals, Inc. MDD US Operations, LLC is the exclusive licensee and distributor of ONAPGO in the United States and Its territories. ONAPGO is a trademark of BRITUSWIP LTD. ©2025 MDD US Operations, LLC, a subsidiary of Supernus Pharmaceuticals, Inc. For more information, go to ONAPGO.comor call 1-833-366-2746 RA-ONG-PPI-V1
-
Patient
INSTRUCTIONS FOR USE
ONAPGO™ (on-AP-goh)

This Instructions for Use contains information on how to use the ONAPGO Infusion System.
Please see the Important Safety Information, Full Prescribing Information, and Patient Information for ONAPGO.
MDD US Operations, LLC
Table of Contents Table of Contents 3 ONAPGO Infusion System 5 Indication for Use 5 Introduction to the Infusion System 5 Important Information 6 Pump Warnings and Precautions 6 Pump Kit 9 Pump Diagram 9 Pump Screen Icons 11 Other Supplies Provided Separately 12 Storage 13 Glass Drug Cartridge Storage 13 Pump Storage 13 Important Information You Need to Know Before Infusing ONAPGO 13 Important Safety Information 13 Check Pump Settings 14 Step 1: Check Pump Settings 14 Setup Set Up the Pump 23 Step 2: Wash Your Hands and Gather Supplies 23 Step 3: Assemble Plastic Cartridge Holder 25 Step 4: Prime the Infusion Line 33 Prepare the Infusion Site 38 Step 5: Select and Clean the Infusion Site 38 Step 6: Connect the Cannula to the Body 39 Connect the Infusion Line to Body 40 Step 7: Connect the Infusion Line to the Cannula 40 Start the Infusion 41 Step 8: Start the Infusion 41 Wear the Pump 43 Step 9: Wear the Pump 43 Reading the Pump Screen 45 ON (Infusing Screen) 45 Paused 46 Primed 46 Off 46 Extra Dose / Pause / End Give a + Dose (Extra Dose) 51 Step 10: Give an Extra Dose 51 Another Way of Giving an Extra Dose 53 Understanding the Extra Dose Features 54 Pause and Resume the Infusion 55 Step 11: Pausing the Infusion 55 Step 12: Resuming the Infusion 57 Ending the Infusion 59 Step 13: Ending the Infusion Early 59 Step 14: Ending the Infusion Regularly 64 Disconnecting in an Emergency 68 Step 15: Disconnecting in an Emergency 68 Pump Care Disposal 73 Glass Drug Cartridge, Plastic Cartridge Holder, and Infusion Line Disposal 73 Pump Disposal 73 Cleaning and Disinfecting the Pump 74 Step 16: Clean and Disinfect the Pump 75 Maintenance and Repair 75 Replacing the Battery 81 Step 17: Replacing the Battery 81 Alarms & Errors Alarms and Troubleshooting 91 Error Codes 91 Troubleshooting: If Your Infusion Line Did Not Prime 98 Symbols Used on the ONAPGO Pump 99 Technical characteristics of the Pump 100 Electromagnetic Compatibility 102 Tests of Accuracy 104 Limited Warranty 105 Contact & Support 105 ONAPGO Infusion System
Indication for Use The ONAPGO Infusion Pump is for the subcutaneous infusion of ONAPGO (apomorphine hydrochloride). ONAPGO is a prescription medicine used to treat motor fluctuations in adults with advanced Parkinson's disease (PD).
Only use the ONAPGO Infusion Pump if you have received instruction and training on how to correctly and safely use it. If you cannot use ONAPGO by yourself, contact your healthcare provider.
Only the accessories supplied by CANÈ S.p.A. (plastic cartridge holder and supplies provided in the infusion pump kit) may be used in conjunction with this pump. The pump must be set (programmed) by a trained healthcare provider or a trained nurse provided by the company (nurse navigator).
Introduction to the Infusion System Below are common terms used throughout this Instructions for Use.
- Pump:controls the rate and amount of ONAPGO delivered to the body. It is programmed by a healthcare provider based on the patient's prescription. The pump displays the status of medicine delivery.
- Priming:refers to the task of removing all air from the infusion line (fluid pathway) before the infusion line is connected to the body.
- Flow Rate:the constant flow of ONAPGO given to the body over an extended period of time. The flow rate is expressed as milligrams per hour (mg/hr).
- Infusion Time:the total time that ONAPGO is infused per day.
-
Extra Dose (+ Dose):a boost of ONAPGO (bolus dose) given over a short period of time in addition to the prescribed flow rate. Your healthcare provider will decide the number of extra doses that you could have each day. Your healthcare provider might prescribe an extra dose to be used as follows:
- when you start the infusion each day, which can help the medicine work faster.
- When you restart your medicine after not having it for 1 hour or more.
- if you have an OFF episode during an infusion.
- Extra Dose (+ Dose) Lock:the amount of time before another extra dose can be given.
- Pump Settings:the patient's prescribed Flow Rate, Maximum Infusing Time Per Day, + Dose Quantity, + Dose Volume, and + Dose Lock. These settings can only be changed by a healthcare provider or clinical nurse navigator.
Important Information - Read this entire Instructions for Use before using the infusion system.
If any of the information is not clear, or if you have any questions, please contact Customer Support at 1-833-3ONAPGO (1-833-366-2746) - Understanding how to use the pump and the potential alarms will help you avoid harm.
- You may hear motor noise when the pump is On and delivering ONAPGO. This noise is normal and means the pump is working correctly.
-
The pump screen falls asleep (goes dark) after 30 seconds of inactivity. Press the UP ARROW button (
 ) to wake up the screen.
) to wake up the screen.
Pump Warnings and Precautions Failure to follow the warnings and precautions below could cause return of your symptoms, damage to the pump, serious injury, or may lead to death in rare cases.
 WARNINGS:
WARNINGS:
- Always check that the pump settings match the prescription before starting an infusion.
See the Check Pump Settingssection on pages 14 to 18 for how to check the pump settings to make sure they are correct. If any of these settings do not match the prescription, contact your healthcare provider and/or clinical nurse navigator. - The patient should receive no more than 98 mg of ONAPGO every 24 hours. This corresponds to one complete cartridge per day. Only use the pump with ONAPGO (apomorphine hydrochloride). ONAPGO comes in a glass cartridge that contains 98 mg/20 mL (4.9 mg/mL) of medicine. Only use the pump system within the temperatures on page 13 and other conditions for use in this Instructions for Use. Using the medicine and pump outside of these conditions may result in a system alarm.
- Only use the pump with the supplied plastic cartridge holder. Any other cartridge holder could damage the Pump and cause harm. Any non- approved cartridge holder voids warranty. MMD US Operations, LLC or its manufacturer is not responsible for any damages or injuries that happen if a different cartridge holder is used.
- Use the infusion set that is provided by your specialty pharmacy. The use of an inappropriate infusion set can cause loss or leakage of drug. Use a new infusion set at the beginning of every infusion. Ask your healthcare provider and/or clinical nurse navigator for more information.
- Do notprime the infusion line when it is connected to the body.
- Do notinsert the cannula into a blood vessel.
- If the medicine is cloudy, green, or contains particles, throw the glass drug Cartridge in a sharps container. (See the Disposalsection on page 73 for information on how to safely throw away ONAPGO). Get a new cartridge for your infusion.
- Clean the top of the glass drug cartridge with a new alcohol wipe before attaching the plastic cartridge holder.
- Always clean the selected infusion site with a new alcohol wipe. Make sure the site is dry before going on to the next step.
- Before starting your infusion, check for any leaks. Do notuse the pump if there are leaks. Contact Customer Support at 1-833-3ONAPGO (1-833-366-2746).
- Be careful when handling the Infusion Set to prevent needle sticks.
- Supplies included with your pump could pose a choking hazard for small children. Make sure that the pump and all supplies are kept away from small children.
- Supplies included with your pump (such as the infusion line) could pose a strangulation hazard. Arrange the infusion line to minimize the risk of strangulation. Make sure that these parts are stored in a secure place when not in use.
- Throw away (discard) the battery if it becomes damaged. Use a new, unopened battery.
See Replacing the Batterysection on pages 81 to 85 (Steps 17.1 through 17.9) for instructions on how to replace the battery. - The pump can detect a blockage in the line. If a partial blockage occurs, the pump will work the way it should until the line has a full blockage. An alarm will sound if the line has a full blockage.
- Be sure to disconnect the infusion line from the body before clearing a blockage alarm. If a blockage alarm is cleared while the infusion line is still attached, you may receive a small amount of additional medicine. See the Alarms and Troubleshootingsection on page 91.
- Confirm that the plastic cartridge holder and infusion line packaging are sealed and have not been damaged. If the packaging looks damaged, throw it away in a sharps container, and do not use it for your infusion. See the Disposalsection on page 73 for information on how to safely throw away ONAPGO. Use a new, undamaged product.
- If the battery becomes hot, do not continue with your infusion and call Customer Support at 1-833-3ONAPGO (1-833-366-2746).
- If your glass drug cartridge breaks while assembled in the plastic cartridge holder, do not separate it from the plastic cartridge holder. Disconnect the assembled cartridge from the pump and throw both away in a sharps container. See the Disposalsection on page 73 for information on how to safely throw away ONAPGO.
- Do notsnag or kink the infusion line while wearing the pump. Snagging or kinking the infusion line can cause a blockage (occlusion) or an interruption to the infusion.
- Always check the expiration date on the infusion set, plastic cartridge holder and glass drug cartridge prior to use. Do notuse expired materials.
- Do notuse expired or rechargeable batteries with the pump.
- If the glass drug cartridge is dropped, it should not be used and should be thrown away in a sharps container.
- If the pump gets wet, pause the infusion, disconnect the infusion line from the body, and store the pump in a dry place. This includes when swimming, showering, or excessively sweating.
- If your ONAPGO pump has expired, do not use it. Call your healthcare provider and/or clinical nurse navigator for a replacement of your ONAPGO pump.
 PRECAUTIONS:
PRECAUTIONS:
- Do notuse sharp objects to press the pump buttons. Only use your fingertips to press the pump buttons.
- Do notuse a damaged pump. If you have any concern about the correct functioning of the pump, or how to respond to an error, contact Customer Support at 1-833-3ONAPGO (1-833-366-2746).
- Read the infusion set Instructions for Use before using the infusion line.
- All pump settings, including sounds, are locked for your safety.
- Do notattempt to change the pump's prescribed settings. These can only be changed by a healthcare provider and/or clinical nurse navigator.
- Do notdisconnect the infusion line from the plastic cartridge holder while the pump is infusing.
- Only clean the infusion site with an alcohol wipe. Do not clean the infusion site with or apply disinfectants, perfumes, deodorants, or lotions to it.
- Free-flow is if gravity causes the drug to come out of the cartridge. The pump's pusher is made to have a secure connection with the rubber plunger in the cartridge to prevent free flow of the drug.
Pump Kit (Figure A) 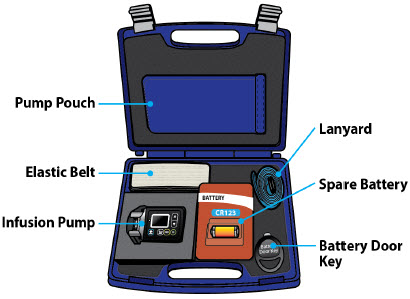
Pump Diagram (Figures B, C, and D) 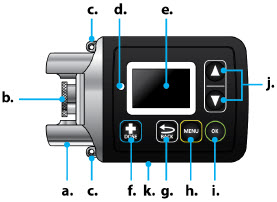 Figure B
Figure B
Figure D Figure C
Figure CAll buttons will respond with a single press. If the screen is dark, press the UP ARROW button (
 ) to wake up the screen.
) to wake up the screen.
Item Pump Feature Description a Cartridge Connection Area where assembled glass drug cartridge and plastic cartridge holder is attached to the pump. b Pump Pusher Pushes ONAPGO from the glass drug cartridge into the infusion line. c Neck Lanyard Clips Slots to clip on the lanyard. d LED Lights up when an error cannot be displayed on the screen. e Screen Where messages and menus appear. f 
+DOSE button gives an extra dose (+ dose) (if prescribed). g 
BACK button allows you to return to a previous screen, menu, or cancel an action. h 
MENU button allows you to access the menus. i 
OK button allows you to confirm menu selections or go on to the next value. j 
UP and DOWN ARROW buttons allow you to scroll through menus. k Battery Door Door to access the battery. Pump Screen Icons The following table shows Icons that appear on the pump screen.
Icon Name Description 
Back Arrow The back arrow is shown when checking the pump settings. This icon is used to show that pressing the BACK button will return to a previous screen (Item 'g' in Figure B). 
Caution Caution is shown when an error or alarm is occurring. 
Check Mark A check mark is shown when the pump successfully completes a task. 
Droplet A blinking droplet is shown on the Infusing Screen when an infusion is on. 
Dead Battery The dead battery is shown when the pump battery is dead and must be replaced. 
Low Battery The Low Battery is shown when the pump battery is low and should be replaced before the next infusion. 
OK Arrow The OK Arrow is shown when checking the pump settings. This icon is used to show that pressing the OK button will confirm a selection or go on to the next value (Item 'i' in Figure B). 
Padlock Padlock is shown when the +Dose (extra dose) is not available. 

Pause Pause is shown when the infusion is paused. 
+Dose (Extra Dose) A +refers to the +Dose (extra dose) function. Other Supplies Provided Separately Additional supplies not in the pump kit include (Figure E):
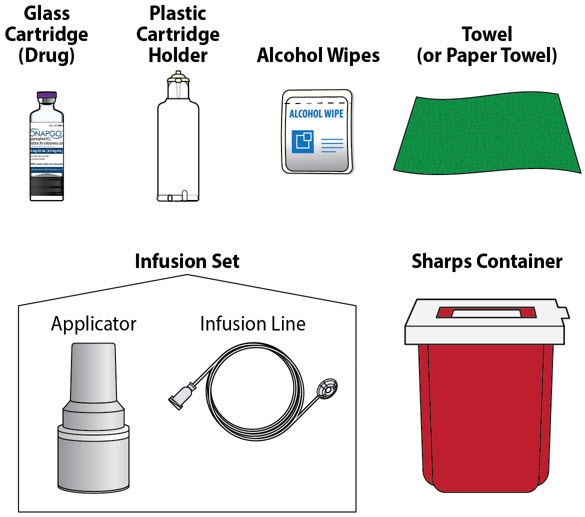
Note:The plastic cartridge holder, glass drug cartridge and infusion set parts are sterile. Figure E Storage
Glass Drug Cartridge Storage Store the glass drug cartridges at room temperature between 68°F to 77°F (20°C to 25°C). Store in original container.
Pump Storage If the pump will not be used for 1 month or more, remove the battery. See Replacing the Batterysection on pages 81 to 85 (Steps 17.1 through 17.9) for instructions on how to remove the battery.
Store the pump in a dry place between -13°F to 158°F (-25°C to +70°C).
Keep the pump away from:
- Children and pets.
- Sources of heat or cold such as radiators, burners, stoves, freezer, or other heat or cold sources. Ask your healthcare provider or clinical nurse navigator if you are not sure.
- Direct sunlight.
- Strong electromagnetic fields such as magnets, speakers, mobile devices.
- Ionizing radiation, a type of device for radiotherapy or diagnostic radiology.
- Ultrasound devices.
- Magnetic Resonance Imaging (MRI) devices. The pump should not be used with an MRI.
If you are not sure about what to keep your pump away from, please contact your Customer Support at 1-833-3ONAPGO (1-833-366-2746).
Important Information You Need to Know Before Infusing ONAPGO
Important Safety Information This Instructions for Use is for patients, caregivers, and healthcare providers or clinical nurse navigators and describes how to use the infusion system.
- The Instructions for Use are important for the safe and correct use of this infusion system.
- Read the entire Instructions for Use before using the infusion system.
Check Pump Settings
Step 1: Check Pump Settings Confirm your pump settings match your prescription by doing the following steps.

Do notstart an infusion if any of the pump settings do not match your prescription. If this happens, contact your healthcare provider or clinical nurse navigator. Step 1.1
Look at the pump.
If the pump screen is dark, press the Up Arrow button (
 ) to wake up the screen (Figure F).
) to wake up the screen (Figure F).
 Figure F
Figure F
You will then see the Infusion Off screen (Figure G). 
Figure G
Step 1.2
Press the MENU button (
 ) to find the pump settings (Figure H).
) to find the pump settings (Figure H).
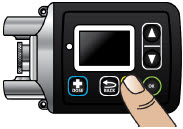
Figure H
Step 1.3
Highlight Settings on the screen using the DOWN ARROW button (
 ) (Figure I).
) (Figure I).
 Figure I
Figure I
Press the OK button (
 ) to begin reviewing the pump settings (Figure J).
) to begin reviewing the pump settings (Figure J).
 Figure J
Figure J
Step 1.4
Make sure the flow rate (mg/hr) matches your prescription (Figure K).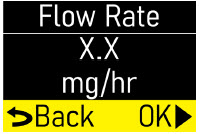 Figure K
Figure K
Press the OK button (
 ) to move to the next screen (Figure L).
) to move to the next screen (Figure L).
 Figure L
Figure L
Step 1.5
Make sure the infusion time (hr) matches your prescription (Figure M).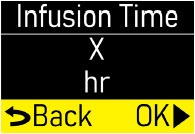 Figure M
Figure M
Press the OK button (
 ) to move to the next screen (Figure N).
) to move to the next screen (Figure N).
 Figure N
Figure N
Step 1.6
Make sure the number of +Doses/ Day (extra doses/day) matches your prescription (Figure O).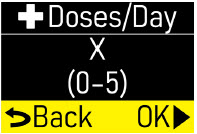 Figure O
Figure O
Press the OK button (
 ) to move to the next screen (Figure P).
) to move to the next screen (Figure P).
 Figure P
Figure P
Step 1.7
Make sure the +Dose (extra dose) amount (mg) matches your prescription (Figure Q).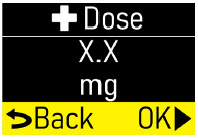 Figure Q
Figure Q
Press the OK button (
 ) to move to next screen (Figure R).
) to move to next screen (Figure R).
 Figure R
Figure R
Step 1.8
Make sure +Dose Lock (extra dose lock) time (hr, min) matches your prescription (Figure S).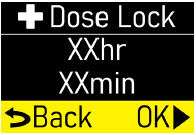 Figure S
Figure S
Press the OK button (
 ) to move to next screen (Figure T).
) to move to next screen (Figure T).
 Figure T
Figure T
Step 1.9
You will see the software version (Figure U). Figure U
Figure U
Press the OK button (
 ) to move to the next screen (Figure V).
) to move to the next screen (Figure V).
 Figure V
Figure V
Step 1.10
Exitwill be highlighted on the Menu screen (Figure W). Figure W
Figure W
Press the OK button (
 ) to exit the menu (Figure X).
) to exit the menu (Figure X).
 Figure X
Figure X
You will then see the Infusion Off screen (Figure Y). Set the pump aside on a clean surface and continue to the Set up the Pump section.  Figure Y
Figure Y
Setup Within this section you will find: Set up the Pump 23 Prepare the Infusion Site 38 Connect the Infusion Line to Body 40 Start the Infusion 41 Wear the Pump 43 Reading the Pump Screen 45 Set Up the Pump
Step 2: Wash Your Hands and Gather Supplies Step 2.1
Go to a sink and wash your hands with soap and water, then dry your hands (Figure Z). Figure Z
Figure Z
Step 2.2
Prepare a clean, flat surface such as a table to work on (Figure AA). Figure AA
Figure AAStep 2.3
Take the blue case and open it (Figure AB). Gather the additional supplies not included in the case (Figure AC).Infusion System Kit Supplies Included in the Case (Figure AB):
- Pump Pouch
- Elastic Belt
- Pump
- Lanyard
- Spare Battery
- Battery Door Key
Additional Supplies not Included in the Case (Figure AC):
- Glass drug Cartridge (Sterile)
- Plastic Cartridge Holder (Sterile)
- Infusion Set parts (Sterile)
- Alcohol Wipes
- Towel (or Paper Towels)
- Sharps Container. See the DisposalSection on page 73 for information on how to safely throw away ONAPGO.

Do notuse other drug cartridges with the pump. The pump must only be used with an ONAPGO glass drug cartridge containing apomorphine hydrochloride 98 mg/20 mL (4.9mg/mL). 
Do notuse other cartridge holders with the pump. The Pump must only be used with the plastic cartridge holder that is provided. 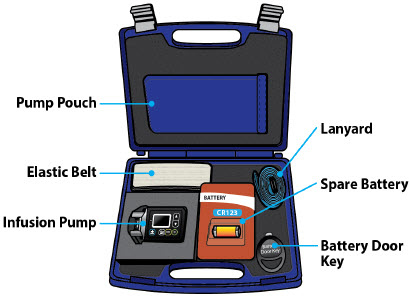
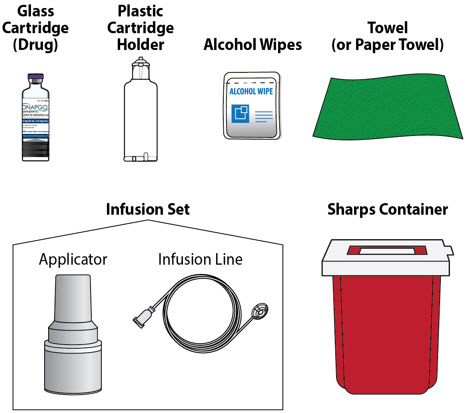
Step 3: Assemble Plastic Cartridge Holder Step 3.1 Prepare Plastic Cartridge Holder
Look at the plastic cartridge holder package. Make sure the expiration date (EXP) has not passed (Figures AD and AE). Figure AD
Figure AD
If the expiration date has passed:
Throw the plastic cartridge holder away in a sharps container and get a new plastic cartridge holder. See the Disposalsection on page 73 for information on how to safely throw away ONAPGO. Figure AE
Figure AE
Remove the plastic cartridge holder from its package (Figure AF).  Figure AF
Figure AF
Step 3.2 Prepare Infusion Line
Look at the infusion line package. Make sure the expiration date has not passed (Figure AG).
If the expiration date has passed:
Throw the infusion line away in a sharps container and get a new infusion line. See the Disposalsection on page 73 for information on how to safely throw away ONAPGO. Figure AG
Figure AG
Remove the infusion line from its package and tear off the paper tab.
Unravel the infusion line so it is straight (Figure AH).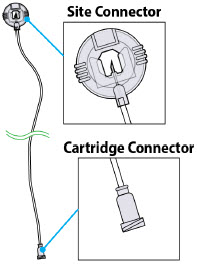 Figure AH
Figure AH
Step 3.3 Connect Infusion Line and Plastic Cartridge Holder
Find the cartridge connector end of the infusion line (Figure AI).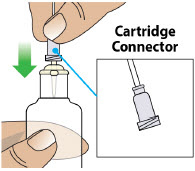 Figure AI
Figure AI
Place the cartridge connector end of the infusion line over the tip of the plastic cartridge holder and turn the cartridge connector until it is snug (Figures AJ and AK).
Note:It only takes a small movement to secure the parts together.
Do not connect the infusion line to the body at this time.You must prime the infusion line before connecting it to your body.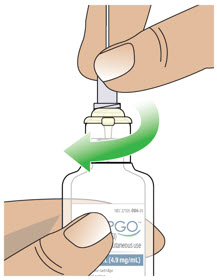 Figure AJ
Figure AJ
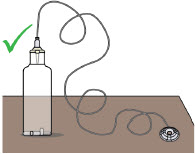 Figure AK
Figure AK
Step 3.4 Prepare Glass Drug Cartridge
Remove the glass drug cartridge from its carton and look at the label. Make sure the expiration date has not passed on the glass drug cartridge (Figures AL and AM). Figure AL
Figure AL
If the expiration date has passed:
Throw the glass drug cartridge away in a sharps container and get a new glass drug cartridge. See the Disposalsection on page 73 for information on how to safely throw away ONAPGO. Figure AM
Figure AM
Step 3.5 Check Medicine
Look at the glass drug cartridge. Make sure the liquid medicine inside the glass drug cartridge is clear, almost colorless, and free from particles (Figure AN). Figure AN
Figure AN
Important Information

If the medicine is cloudy, discolored, or contains particles,throw away the glass drug cartridge in a sharps container and get a new one. See the Disposalsection on page 73 for information on how to safely throw away ONAPGO. Step 3.6 Remove Flip Cap from Glass Drug Cartridge
Hold the glass drug cartridge and remove the flip cap (Figure AO). You may need to push hard to remove the flip cap.
You can throw the flip cap away in your household trash.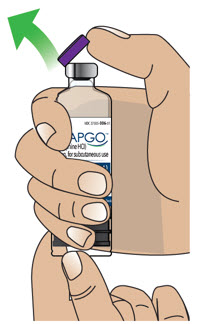 Figure AO
Figure AO
Step 3.7 Clean Glass Drug Cartridge
Clean the top of the glass drug cartridge with a new alcohol wipe (Figure AP).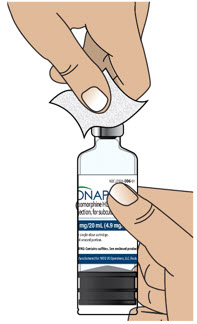 Figure AP
Figure AP
Step 3.8 Place Glass Drug Cartridge
Place the glass drug cartridge on a flat surface on top of a towel or paper towel (Figure AQ). Figure AQ
Figure AQ
Step 3.9 Attach Plastic Cartridge Holder to Glass Drug Cartridge
Take the plastic cartridge holder. Place the plastic cartridge holder, with the infusion line attached, over the glass drug cartridge and push all the way down (Figures AR and AS).
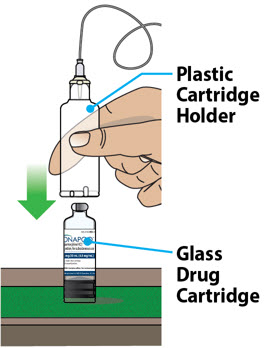 Figure AR
Figure AR
Note:After letting go, the plastic cartridge holder may come up a bit so that it is not even with the bottom of the glass drug cartridge. This is normal.
A small amount of liquid medicine might come out of the glass drug cartridge. This is normal.
You have now created an "assembled cartridge" (Figure AT).
Note:Change the cartridge, cartridge holder, and administration set daily or between uses.
Note:If the infusion line is dropped or dirty, throw away (discard) the infusion line.
Warning:Do not puncture the cartridge with anything but the specified plastic cartridge holder.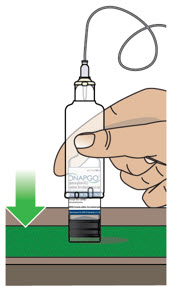 Figure AS
Figure AS
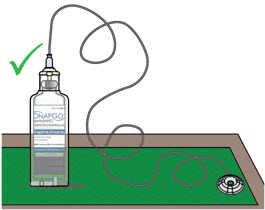 Figure AT
Figure AT
Step 3.10 Place Assembled Cartridge on Pump
Hold the pump upright as shown (Figure AU).
Note:There are ridges at the bottom of the plastic cartridge Holder. You will use these ridges in the next step to align the assembled cartridge with the pump. Figure AU
Figure AU
Take the assembled cartridge. Position the assembled cartridge above the pump with the ridges facing you (Figure AV). 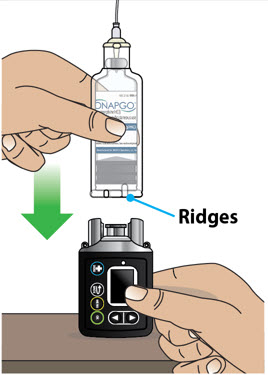 Figure AV
Figure AV
Place the assembled cartridge onto the pump, as shown, with the ridges showing in the pump opening (Figure AW). The assembled cartridge should sit flush against the pump with no gap. 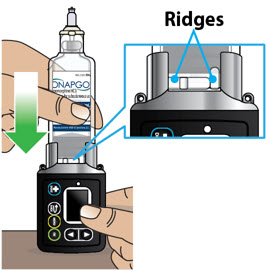 Figure AW
Figure AW
Step 3.11 Attach Assembled Cartridge to Pump
Push downthe assembled cartridge firmly and twistuntil you feel it lock into place and the ridges are no longer showing (Figure AX).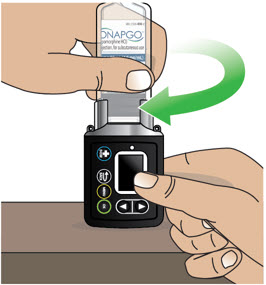 Figure AX
Figure AX
Look at the Pump opening. - If you only see a smooth surface with no gap between the assembled cartridge and the pump, then the assembled cartridge is correctly attached (Figure AY) and you can continue to Step 4.
- If you see any portion of the ridges in the space (Figure AZ), then push down firmly again as you twist the assembled cartridge until the ridges are no longer showing (Figure AY).
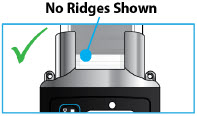 Figure AY
Figure AY
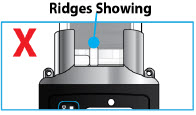 Figure AZ
Figure AZStep 4: Prime the Infusion Line Priming the infusion line (Figure BA) pushes liquid through the system to remove all air from the glass drug cartridge and infusion line. All air must be removed from the system before the infusion line is connected to the infusion site on the body.
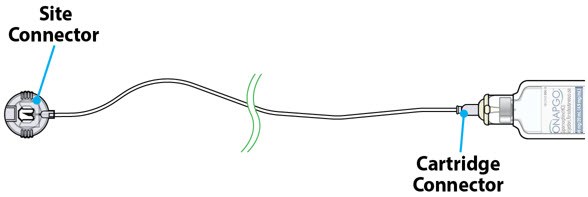

Read the Instructions for Use for the infusion set before using the infusion line. 
Do notprime the infusion line when it is connected to the infusion site on the body. Step 4.1
Take the site connector end of the infusion line and place it on a towel (Figure BB). Figure BB
Figure BB
Step 4.2
Take the pump. Press the UP ARROW button ( ) to wake up the screen (Figure BC).
) to wake up the screen (Figure BC).
 Figure BC
Figure BC
You will then see the Infusion Off screen (Figure BD).  Figure BD
Figure BDStep 4.3
Press the MENU button ( ) to enter the pump menu (Figure BE).
) to enter the pump menu (Figure BE).
 Figure BE
Figure BE
Step 4.4
The Primeoption will be highlighted on the Menu screen (Figure BF). Figure BF
Figure BF
Press the OK button (
 ) to continue (Figure BG).
) to continue (Figure BG).
 Figure BG
Figure BG
Step 4.5
You will see the Ready to Prime screen (Figure BH). Figure BH
Figure BH
Press the OK button (
 ) to prime the infusion line (Figure BI).
) to prime the infusion line (Figure BI).
Note:Make sure that the site connector is held over the towel and not attached to the body. During priming, you will be looking for drops to come out of the site connector. Figure BI
Figure BI
You will then see the Priming screen flashing (Figure BJ). This means that the pump is priming.  Figure BJ
Figure BJ
During Priming…
Check the connector end of the infusion line for drops of liquid medicine (Figure BK).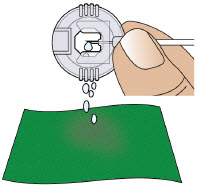 Figure BK
Figure BKImportant Information

Do notallow the medicine to come into contact with your clothing, carpeting, or furniture, as it can stain. Step 4.6
When priming is done you will see this screen (Figure BL). Figure BL
Figure BL
After priming…
Check that all air gaps or large air bubbles are removed from the line (Figure BM).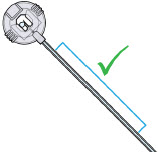 Figure BM
Figure BM
Press the OK button (
 ) for menu options (Figure BN).
) for menu options (Figure BN).
 Figure BN
Figure BN
Step 4.7
Did you see drops and confirm no large air bubbles or gaps in the line?-
If Yes, Press OK button (
 ) to Continue (Figures BO and BP).
) to Continue (Figures BO and BP).
-
If No, Highlight Repeat Primeon the screen using the DOWN ARROW button (
 ).
).
Then press the OK button ( ) to prime again.
) to prime again.
If after two priming attempts the line is still not primed, see page 98 for troubleshooting information.
 Figure BO
Figure BO
 Figure BP
Figure BP
Step 4.8
You will see a screen that says Wait! Connect Line to Body (Figure BQ). Figure BQ
Figure BQ
Put down the pump(Figure BR) and follow the next sections to "Prepare the Infusion Site" and "Connect the Infusion Line to Body".  Figure BR
Figure BRPrepare the Infusion Site
You will insert the cannula (a small tube that allows the medicine to be delivered) into the selected infusion site.
Step 5: Select and Clean the Infusion Site Step 5.1
Select one of the following infusion sites (see blue shaded areas in Figure BS):- Lower stomach (Abdomen), at least 2 inches away from belly button
- Top of thigh
- Lower back
- Upper back (only to be used when a caregiver or healthcare provider is preparing the infusion).
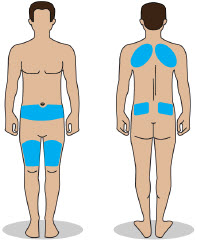 Figure BS
Figure BSImportant Information

Change the infusion site every day (rotate sites) to avoid skin-related problems, such as bumps or nodules. 
Do notselect an infusion site that is bruised, has bumps or nodules, or is irritated. 
Do notselect an infusion site other than those listed and shown in Figure BS. Step 5.2
Get an alcohol wipe. Clean the selected infusion site with a new alcohol wipe (Figure BT).
Make sure the area is dry before moving on to Step 6.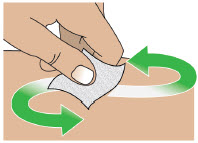 Figure BT
Figure BTNote:If the infusion line is dropped or dirty, throw away (discard) the infusion line.
Step 6: Connect the Cannula to the Body Step 6.1
Following the infusion set Instructions for Use, insert the cannula into the cleaned infusion site (Figure BU).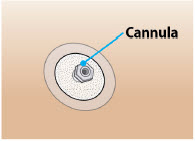 Figure BU
Figure BU
Read the Instructions for Use for the infusion set before using the infusion line. 
Do notinsert the cannula into a blood vessel. 
Do notinsert the cannula into a bump or nodule, as it can result in a blockage (occlusion). Connect the Infusion Line to Body
Step 7: Connect the Infusion Line to the Cannula Step 7.1
Take the site connector end of the infusion line. Pinch the sides of it and pull on the end of the site connector to open it (Figure BV). Figure BV
Figure BV
Step 7.2
Connect the primed infusion line to the cannula on the body by placing the site connector over the cannula and then closing it (Figures BW and BX). For more information, follow the Instructions for Use provided with the infusion set.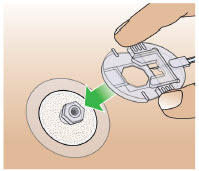 Figure BW
Figure BW
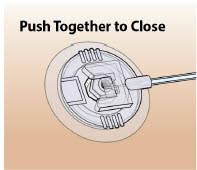 Figure BX
Figure BXStart the Infusion
Step 8: Start the Infusion Step 8.1
Pick up the pump.
Press the UP ARROW button (
 ) to wake up the screen (Figure BY).
) to wake up the screen (Figure BY).
 Figure BY
Figure BY
You will see a screen that says Connect Line to Body (Figure BZ).  Figure BZ
Figure BZ
Now that you have connected the line to your body, press the OK button (
 ) (Figure CA).
) (Figure CA).
 Figure CA
Figure CA
You will see a screen that says Confirm Line Connected (Figure CB). 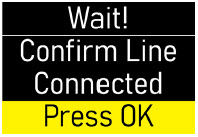 Figure CB
Figure CB
Make sure that the site connector is attached to the body.
Then press the OK button ( ) (Figure CC).
) (Figure CC).
 Figure CC
Figure CC
Step 8.2
You will see a screen that says Start Infusion (Figure CD). Figure CD
Figure CD
When ready, press the OK button (
 ) to start the infusion (Figure CE).
) to start the infusion (Figure CE).
 Figure CE
Figure CE
You will then see the Infusion Started screen. The checkmark at the bottom of the screen means that the infusion has successfully started (Figure CF).  Figure CF
Figure CF
After the infusion has started, you will see the Infusing Screen. The flashing droplet means that the pump is now infusing (Figure CG). Next to the droplet, you will also see the amount of time before the infusion ends.  Figure CG
Figure CGWear the Pump
Step 9: Wear the Pump You must place the pump inside the pump pouch and then attach it to either the elastic belt or the lanyard, whichever you prefer.
Using the Elastic BeltTake the elastic belt and pump pouch. Insert the elastic belt into the loop on the back of the pump pouch (Figure CH). 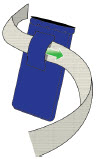 Figure CH
Figure CH
Take the pump. Place the pump inside of the pump pouch (Figure CI). 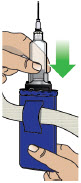 Figure CI
Figure CI
Secure the elastic belt around the waist (Figure CJ). 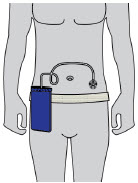 Figure CJ
Figure CJ
Do notsnag or kink the infusion line while wearing the pump. Snagging or kinking the infusion line can cause a blockage (occlusion) or an interruption to the infusion. Using the Neck Lanyard
Take the neck lanyard and pump. Hook the lanyard to the neck lanyard Clips (Figure CK) located on the pump.  Figure CK
Figure CK
Take the pump pouch. Place the pump inside of the pump pouch (Figure CL). 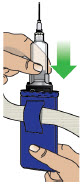 Figure CL
Figure CL
Place the lanyard around patient's neck (Figure CM). 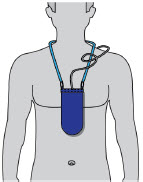 Figure CM
Figure CM
Do notsnag or kink the infusion line while wearing the Pump. Snagging or kinking the infusion line can cause a blockage (occlusion) or an interruption to the infusion. Reading the Pump Screen
There are 4 main modes for the pump: On (Infusing Screen), Paused, Primed, and Infusion Off. Each mode is described below.
ON (Infusing Screen) When the pump is On, and the infusion is running, the following icons and information will be seen (Figure CN): 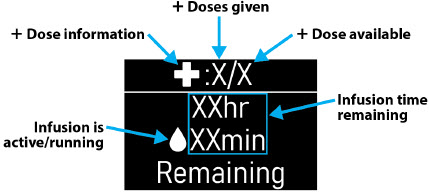 Figure CN
Figure CNWhen the pump is On and you attempt to give an extra dose ( +Dose), the following icon will be seen when the extra dose ( +Dose) is locked out (Figure CO): 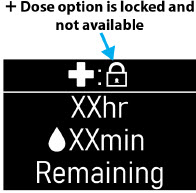 Figure CO
Figure COPaused When the infusion is paused, the following screen will be seen (Figure CP):  Figure CP
Figure CPTo pause the infusion, see the Pausing the Infusioninstructions on page 55. Primed When the pump is primed, but the infusion has not started, the following screen will be seen (Figure CQ):  Figure CQ
Figure CQOFF When the pump is not infusing, the following screen will be seen (Figure CR):  Figure CR
Figure CR+ Dose / Pause / End Within this section you will find: Give a +Dose 51 Pause and Resume the Infusion 55 Ending the Infusion 59 Disconnecting in an Emergency 68 Give a +Dose (Extra Dose)
Step 10: Give an Extra Dose (+ Dose) An extra dose ( +Dose) is only available if it has been prescribed by the healthcare provider. If an extra dose ( +Dose) has NOT been prescribed, skip this section.
Note:An extra dose ( +Dose) can only be given after the pump has been primed and while the pump is infusing.
Step 10.1
Take the pump. Press the UP ARROW button ( ) to wake up the screen (Figure CS).
) to wake up the screen (Figure CS).
 Figure CS
Figure CS
Step 10.2
Press the +Dose button ( ) (Figure CT).
) (Figure CT).
 Figure CT
Figure CT
You will then see the +Dose screen. Press the DOWN ARROW button (
 ) to highlight
Yes(Figure CU).
) to highlight
Yes(Figure CU).
 Figure CU
Figure CU
Step 10.3
Press the OK button ( ) to continue (Figure CV).
) to continue (Figure CV).
 Figure CV
Figure CV
The pump will now give the extra dose ( +Dose ) (Figure CW).  Figure CW
Figure CW
When the extra dose is delivered, you will then see the +Dose Delivered screen with a check mark on the bottom (Figure CX).  Figure CX
Figure CX
The pump will then automatically go back to the Infusing screen (Figure CY).
Note:Administer extra doses ( +Doses) at least 3 hours apart. Administer no more than 3 extra doses a day. Figure CY
Figure CYAnother Way of Giving an Extra Dose (+ Dose) 
Do notrepeat the steps below if you have already given an extra dose using the +Dose button (
 ).
).
Take the pump.
Press the menu button ( ) (Figure CZ).
) (Figure CZ).
 Figure CZ
Figure CZ
Use the DOWN ARROW button (
 ) to highlight Give
+Dose (Figure DA).
) to highlight Give
+Dose (Figure DA).
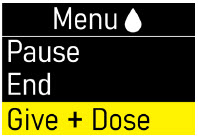 Figure DA
Figure DA
Press the OK button (
 ) to continue (Figure DB).
) to continue (Figure DB).
 Figure DB
Figure DB
You will then see the +Dose screen.
Press the DOWN ARROW button ( ) to highlight Yes (Figure DC)
) to highlight Yes (Figure DC)
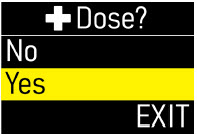 Figure DC
Figure DC
Press the OK button (
 ) to deliver the extra dose (
+Dose)(Figure DD).
) to deliver the extra dose (
+Dose)(Figure DD).
 Figure DD
Figure DDUnderstanding the Extra Dose (+ Dose) Features If you see the +Dose Locked icon (Figure DE) on the home screen, it means that an extra dose is not allowed.
This is because the minimum amount of time has not passed since the last extra dose ( +Dose) was given.
The time between allowable extra doses ( +Doses) is prescribed by the healthcare provider. Figure DE
Figure DE
When the +Dose is locked, you can see the amount of time remaining before another extra dose ( +Dose) can be given, by pressing the +Dose button (
 ) (Figure DF).
) (Figure DF).
 Figure DF
Figure DF
If you see the +Dose None Remaining message (Figure DG), it means the maximum number of extra doses has been reached and none remain for that infusion.  Figure DG
Figure DGPause and Resume the Infusion
If the pump is at risk for getting wet, pause the infusion, disconnect the infusion line from the body, and store the pump in a dry place. This includes swimming, showering, or excessive sweating.
Step 11: Pausing the Infusion Step 11.1
Take the pump. Press the UP ARROW button ( ) to wake up the screen (Figure DH).
) to wake up the screen (Figure DH).
 Figure DH
Figure DH
Step 11.2
Press the MENU button ( ) to enter the pump menu (Figure DI).
) to enter the pump menu (Figure DI).
 Figure DI
Figure DI
Step 11.3
Pause option will be highlighted on the Menu screen (Figure DJ).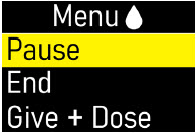 Figure DJ
Figure DJ
Step 11.4
Press the OK button ( ) to pause the infusion (Figure DK).
) to pause the infusion (Figure DK).
 Figure DK
Figure DK
You will then see the Paused screen (Figure DL).  Figure DL
Figure DL
Step 11.5 (If Needed)
If needed, remove the infusion line from your body (Figure DM) while leaving cannula on your skin. There is no need to cover the site connector once removed, as it is self-sealing.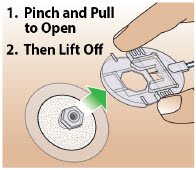 Figure DM
Figure DMStep 12: Resuming the Infusion Step 12.1 (If Needed)
Take the site connector end of the infusion line. Reattach the infusion line to the infusion site (Figure DN). Figure DN
Figure DN
Step 12.2
Take the pump. Press the UP ARROW button ( ) to wake up the screen (Figure DO).
) to wake up the screen (Figure DO).
 Figure DO
Figure DO
�Step 12.3
Press the MENU button ( ) to enter the pump menu (Figure DP).
) to enter the pump menu (Figure DP).
 Figure DP
Figure DP
Step 12.4
Resume option will be highlighted on the Menu screen (Figure DQ). Figure DQ
Figure DQ
Step 12.5
Press the OK button ( ) to resume the infusion (Figure DR).
) to resume the infusion (Figure DR).
 Figure DR
Figure DR
You will then see the Infusion Resumed screen with a check mark on the bottom (Figure DS).  Figure DS
Figure DSThe pump will automatically return to the Infusing Screen (Figure DT).  Figure DT
Figure DTEnding the Infusion
The infusion will run for the programmed amount of time, or it can be ended early.
Step 13: Ending the Infusion Early Step 13.1
Take the pump. Press the UP ARROW button ( ) to wake up the screen (Figure DU).
) to wake up the screen (Figure DU).
 Figure DU
Figure DU
Step 13.2
Press the MENU button ( ) to enter the pump menu (Figure DV).
) to enter the pump menu (Figure DV).
 Figure DV
Figure DV
Step 13.3
Press the DOWN ARROW button ( ) to highlight
End(Figure DW).
) to highlight
End(Figure DW).
 Figure DW
Figure DW
Press the OK button (
 ) to end the infusion (Figure DX).
) to end the infusion (Figure DX).
 Figure DX
Figure DX
Step 13.4
Press the DOWN ARROW button ( ) to highlight
Yes(Figure DY).
) to highlight
Yes(Figure DY).
 Figure DY
Figure DY
Press the OK button (
 ) to select
Yes(Figure DZ).
) to select
Yes(Figure DZ).
 Figure DZ
Figure DZ
You will then see the Stop! Remove Line From Body screen (Figure EA).
Do notpress the OK button at this time. Figure EA
Figure EA
Step 13.5
Remove the infusion line from the body (Figure EB). Figure EB
Figure EB
Step 13.6
Press the OK button ( ) to confirm that the infusion line has been removed from the body (Figure EC).
) to confirm that the infusion line has been removed from the body (Figure EC).
 Figure EC
Figure ECYou will then see the Ending Infusion screen, indicating the ending of the infusion (Figure ED). It may take several minutes to retract the pusher and end the infusion.  Figure ED
Figure ED
When the pump has ended the infusion, you will see the Infusion Off screen (Figure EE).  Figure EE
Figure EENote:If you press the back (
 ) button as the infusion is ending, the pusher reset will pause. Press the OK button (
) button as the infusion is ending, the pusher reset will pause. Press the OK button (
 ) to resume ending the infusion and resetting the pusher.
) to resume ending the infusion and resetting the pusher.
Step 13.7
Remove the cannula from the body (Figure EF).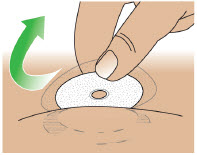 Figure EF
Figure EF
Step 13.8
Take a new alcohol wipe. Clean the infusion site with a new alcohol wipe (Figure EG).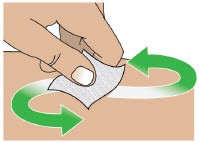 Figure EG
Figure EG
Step 13.9
Gently massage the area of the infusion site to break up any excess fluid buildup. This will help prevent skin nodules or bumps and irritations (Figure EH).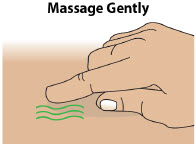 Figure EH
Figure EH
Step 13.10
Take the pump. Twist the assembled cartridge to the left (counterclockwise) and lift it straight up to remove the plastic cartridge holder, glass drug cartridge, and infusion line altogether (Figure EI).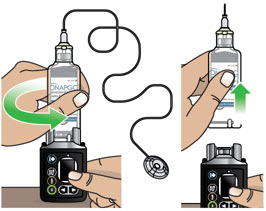 Figure EI
Figure EI
Do notseparate the infusion line or the glass drug cartridge from the plastic cartridge holder. Note:If the plastic cartridge holder detaches from the glass drug cartridge when removing, continue to remove the plastic cartridge holder. Set aside the plastic cartridge holder and remove the glass drug cartridge by pulling it up and off the pump. Continue to Step 13.11.
Step 13.11
Throw away the used Infusion line, glass drug cartridge, plastic cartridge holder, and cannula into a sharps container right away after use (Figure EJ).
Do notthrow away (dispose of) the infusion line, glass drug cartridge, or plastic cartridge holder in your household trash.
See the Disposalsection on page 73 for information on how to safely throw away ONAPGO.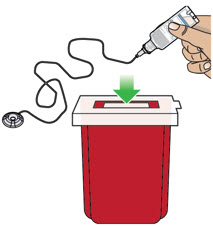 Figure EJ
Figure EJVisually Check the Pump
Right after you use the pump, look it over (visually check) to see if you notice any soiling on the pump.
Wipe off any soiling that you see with isopropyl alcohol. (See the Clean and Disinfect the Pumpsection on page 74.)Step 14: Ending the Infusion Regularly Step 14.1
At 1 hour, 10 minutes, and 5 minutes remaining in the infusion, the pump will give a reminder on the screen and you will hear occasional beeping (Figure EK). Figure EK
Figure EK
You must press the OK button (
 ) to dismiss the message (Figure EL).
) to dismiss the message (Figure EL).
Note:The 10-minute remaining screen is shown in Figure EK as an example. Figure EL
Figure EL
Step 14.2
When the infusion ends, the Ended screen will appear with 0 minutes remaining (Figure EM) and you will hear a beep. Figure EM
Figure EM
Press the OK button (
 ) to select
Yes(Figure EN).
) to select
Yes(Figure EN).
 Figure EN
Figure EN
Step 14.3
You will then see the Stop! Remove Line From Body screen (Figure EO).
Do notpress the OK button at this time. Figure EO
Figure EO
Step 14.4
Remove infusion line from the body (Figure EP). Figure EP
Figure EP
The infusion line must be removed from the infusion site before pressing the OK button. Step 14.5
Take the pump.
Press the OK button ( ) to confirm that the infusion line has been removed from the body (Figure EQ).
) to confirm that the infusion line has been removed from the body (Figure EQ).
 Figure EQ
Figure EQ
The pump will begin to reset, and you will see the Ending Infusion screen (Figure ER). It may take several minutes to retract the pusher and end the infusion.
 Figure ER
Figure ER
Next, you will see the Infusion Off screen (Figure ES).  Figure ES
Figure ES
Note:If you press the back (
 ) button as the infusion is ending, the pusher reset will pause. Press the OK button (
) button as the infusion is ending, the pusher reset will pause. Press the OK button (
 ) to resume ending the infusion and resetting the pusher.
) to resume ending the infusion and resetting the pusher.
Step 14.6
Remove the cannula from the body (Figure ET). Figure ET
Figure ET
Step 14.7
Take a new alcohol wipe. Clean the infusion site with a new alcohol wipe (Figure EU). Figure EU
Figure EU
Step 14.8
Gently massage the area of the infusion site to break up any excess fluid buildup. This will help prevent skin bumps or nodules and irritations (Figure EV). Figure EV
Figure EV
Step 14.9
Take the pump. Twist the plastic cartridge holder to the left (counterclockwise) and lift it straight up to remove the plastic cartridge holder, glass drug cartridge, and infusion line (Figure EW). Figure EW
Figure EW
Do notseparate the infusion line or the glass drug cartridge from the plastic cartridge holder. Note:If the plastic cartridge holder detaches from the glass drug cartridge when removing, continue to remove the plastic cartridge holder. Set aside the plastic cartridge holder and remove the glass drug cartridge by pulling it up and off the pump. Continue to Step 14.10. Step 14.10
Put the used infusion line, glass drug cartridge, plastic cartridge holder, and cannula into a sharps container right away after use (Figure EX).
Do notthrow away (dispose of) the infusion line, glass drug cartridge, or plastic cartridge holder in your household trash.
See the Disposalsection on page 73 for information on how to safely throw away ONAPGO. Figure EX
Figure EXVisually Check the Pump
Right after you use the pump, look it over (visually check) to see if you notice any soiling.
Wipe off any soiling that you see with isopropyl alcohol. (See the Clean and Disinfect the pumpsection on page 74.)Disconnecting in an Emergency
If you are having a medical emergency, follow the steps below to disconnect from the pump.
Step 15: Disconnecting in an Emergency Step 15.1
In the United States, dial 9-1-1 from any telephone for immediate medical assistance (Figure EY). Figure EY
Figure EY
Step 15.2
Remove the infusion line from your body (Figure EZ). Figure EZ
Figure EZStep 15.3
Follow the Ending the Infusion Earlyinstructions on pages 59 to 63.Pump Care Within this section you will find: Disposal 73 Cleaning and Disinfecting the Pump 74 Replacing the Battery 81 Disposal
Glass Drug Cartridge, Plastic Cartridge Holder, and Infusion Line Disposal - After the infusion, put the combined glass drug cartridge, plastic cartridge holder, and infusion set into a sharps container right away after use (Figure FA).
Do notthrow away (dispose of) the glass drug cartridge, plastic cartridge holder, or infusion set in your household trash.
Do notseparate the parts. - If you do not have an FDA-cleared sharps container, you may use a household container that is:
- Made of a heavy-duty plastic.
- Can be closed with a tight-fitting, puncture-resistant lid.
- Upright and stable during use.
- Leak resistant.
- Properly labeled to warn of hazardous waste inside the container.
- When your sharps container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps container. There may be state or local laws about how you should throw away used needles.
- For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do notdispose of your used sharps container in household trash unless your community guidelines permit this.
- Do notrecycle your used sharps container.
 Figure FA
Figure FAPump Disposal At the end of the expected life of the pump, contact Customer Support at 1-833-3ONAPGO (1-833-366-2746) for information and instructions about safe pump disposal.
Cleaning and Disinfecting the Pump
The Pump is a reusable device and should be cleaned and disinfected whenever you see any traces of dirt.
Follow the instructions below and on the following pages for cleaning and disinfecting the pump.

Do notclean and disinfect the pump if it is missing any parts (e.g., battery compartment door) or if you see damage to the front label. 
The pump is not waterproof. Do notimmerse the pump in liquid. 
Do notplace the pump under running water. 
Do notget any liquid inside of the pump. If the pump becomes wet, dry it right away. 
When cleaning the pump, dry your hands oftento avoid the build-up of liquid on the pump surface. 
Do notclean the pump with bleach or harsh (abrasive) detergents. 
Do notsterilize the pump. 
Do notclean the pump in a dishwasher. 
Do notdry the pump in a microwave. Always carefully follow the steps below when cleaning and disinfecting the pump. Step 16.1 Preparing to Clean the Pump Wash and Dry Your Hands
Wash your hands with soap and water, then dry your hands (Figure FB). Figure FB
Figure FB
Set up a Clean Work Area
Set up a clean work area on a clean flat surface (Figure FC). Figure FC
Figure FC
Gather Supplies
Gather the following supplies (available at most supermarkets or pharmacies) (Figure FD):
- Neutral detergent
- Isopropyl alcohol, 70% (rubbing alcohol)
- Purified or distilled water
- Toothbrush with soft bristles
- Several pieces of gauze (at least 8)
- Disposable gloves (to be used during cleaning)
- Disposable cups (to hold water and detergent)
- Absorbent towel (to keep on the table and dry your hands)
 Figure FD
Figure FD
Do notreuse the disposable gauze when cleaning the Pump. Throw away each piece of gauze after use.
Make sure to follow the instructions provided by the manufacturers of the detergent and the isopropyl alcohol.Step 16.2 Thoroughly Clean the Pump with the Toothbrush 
Always clean and disinfect the toothbrush each time you clean the pump, or use a new toothbrush each time you clean the pump. Replace the toothbrush if the bristles get worn or dirty. Wet the Toothbrush
Use the purified or distilled water to slightly moisten the bristles of the toothbrush.Add a Drop of Detergent
Add a drop of detergent to the toothbrush.
Brush Gently Until Clean
Use the toothbrush head to gently brush any areas of the pump that are dirty (Figure FE) until you see the pump is clean.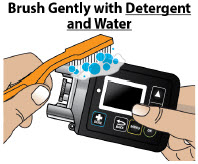 Figure FE
Figure FE
Do notvigorously scrub the pump with the toothbrush. Step 16.3 Dry the Pump Wipe Dry with Gauze
Wipe all areas of the pump with a new dry gauze, to remove excess detergent residue or moisture (Figure FF), then throw away the gauze.
Visually Check the Pump
Visually check that all dirt and residue has been removed.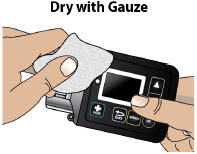 Figure FF
Figure FF
Step 16.4 Thoroughly Clean the Pump with Gauze Dampen a Gauze with Detergent andWater
Dampen a new gauze with detergent and purified or distilled water.
Wipe the Pump with Gauze
Wipe the outer surfaces of the pump using the dampened gauze (Figure FG), then throw away the gauze. Figure FG
Figure FG
16.5 Dampen a New Gauze with Water
Dampen a new gauze with purified or distilled water.
16.6 Wipe the Pump with Gauze
Wipe the outer surfaces of the pump with the dampened gauze to remove any detergent residue (Figure FH), then throw away the gauze. Figure FH
Figure FHStep 16.7 Dry the Pump Wipe Dry with Gauze
Dry all areas of the pump with a new dry gauze (Figure FI), then throw away the gauze.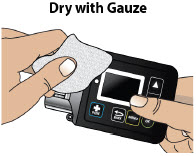 Figure FI
Figure FI
Visually Check the Pump
Visually check the pump for any soiling. Repeat the cleaning process if you see any traces of soiling.Step 16.8 Disinfect the Pump with 70% Isopropyl Alcohol Soak a Gauze with Alcohol
Soak a new gauze with 70% isopropyl alcohol.
Wipe Pump with Gauze for at least 1 Minute
Wipe the outer surfaces of the pump using the soaked gauze for at least one minute(Figure FJ), then throw away the gauze.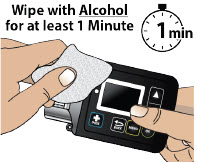 Figure FJ
Figure FJ
Do notimmerse the pump in isopropyl alcohol. Step 16.9 Let Pump Air Dry Let Pump Air Dry for at least 5 Minutes
Set the pump aside on a clean dry surface and let the pump air dry for at least 5 minutes(Figure FK). Figure FK
Figure FK
The isopropyl alcohol must be in contact with the device surfaces for at least 5 minutesto disinfect the pump. Step 16.10 Disinfect the Pump with 70% Isopropyl Alcohol Soak a Gauze with Alcohol
Soak a new gauze with 70% isopropyl alcohol.
Wipe Pump with Gauze again for at least 1 Minute
Wipe the outer surfaces of the pump using the soaked gauze for at least one minute(Figure FL), then throw away the gauze.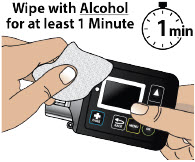 Figure FL
Figure FL
Do notimmerse the pump in isopropyl alcohol. Step 16.11 Let Pump Air Dry Let Pump Air Dry for at least 5 Minutes
Set the pump aside on a clean dry surface and let the pump air dry for at least 5 minutes(Figure FM). Figure FM
Figure FMMake sure Pump is Dry
Make sure the pump is completely dry, and all the isopropyl alcohol has evaporated.
The isopropyl alcohol must be in contact with the device surfaces for at least 5 minutesto disinfect the pump. Step 16.12 Use Water to Remove Alcohol Residue Dampen Gauze with Water
Dampen a new gauze with purified or distilled water. Figure FN
Figure FNWipe the Pump with Gauze
Wipe the outer surfaces of the pump with the dampened gauze to remove any disinfectant residue (Figure FN), then throw away the gauze.Step 16.13 Dry the Pump Wipe Dry with Gauze
Dry all areas of the pump with a new dry gauze (Figure FO), then throw away the gauze.
Visually Check the Pump
Visually inspect the pump to make sure it is clean.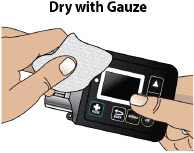 Figure FO
Figure FO
If you see any traces of dirt,repeat the entire cleaning and disinfecting procedure. 
If the pump has not been cleaned for a long period of time, some stains caused by the liquid medicine may not come off during the cleaning and disinfecting process. Maintenance and Repair If the pump is used correctly as outlined in this Instructions for Use, the pump does not need programmed service maintenance from the manufacturer (CANÈ S.p.A. Medical Technology Company). 
If the pump stops working, is damaged, or shows a numbered error code, contact your Specialty Pharmacyor Customer Support at 1-833-3ONAPGO (1-833-366-2746). Replacing the Battery
Always have a replacement lithium CR 123A battery available for use.

Do notreplace the battery while the Infusion Line is connected to your body or during an active infusion as this could cause harm. 
Do notuse a battery other than lithium CR 123A. 
Do notuse expired or rechargeable batteries with the Pump. Step 17: Replacing the Battery To replace the battery, follow these steps:
Step 17.1
You should see the Infusion Off screen (Figure FP). Figure FP
Figure FP
Step 17.2
Take a new 3 volt lithium battery (model CR 123A) (Figure FQ) and the battery door key (Figure FR). Figure FQ
Figure FQ
 Figure FR
Figure FR
Step 17.3
Take the battery door key and pump. Insert the battery door key into the battery door keyhole on the bottom of the pump (Figure FS).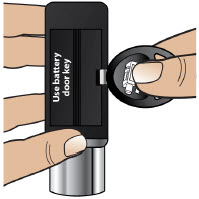 Figure FS
Figure FS
Push the battery door key away from the pump (Figure FT) to open the battery door. 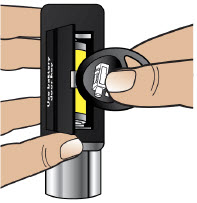 Figure FT
Figure FT
Step 17.4
Pull the battery door up and all the way out so that the ribbon attached to the battery door pops out the battery (Figure FU).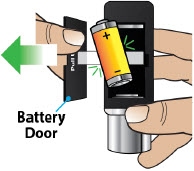 Figure FU
Figure FU
Do notuse any other object to remove the battery. If the battery does not pop out: Hold the pump and the battery door firmly in one hand (Figure FV) and 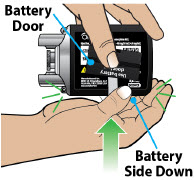 Figure FV
Figure FV
Strike the palm of your other hand with the pump, to jolt the battery from the compartment (Figure FW). 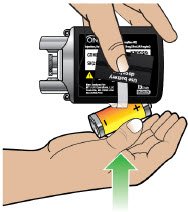 Figure FW
Figure FW
Step 17.5
Throw away the expired battery per local guidelines (Figure FX).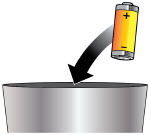 Figure FX
Figure FX
Step 17.6
Wait 10 seconds before inserting a new battery (Figure FY). This allows the pump to reset. Figure FY
Figure FY
Step 17.7
Take your new, unopened 3 volt lithium battery, model CR 123A (Figure FZ).
Open the package. Figure FZ
Figure FZ
Step 17.8
Take the pump. Insert the new battery by placing it on top of the ribbon with the negative end ( -) of the battery towards the silver side of the pump where the plastic cartridge holder is attached, and the positive end ( +) towards the flat end of the pump (Figure GA).
Note:The battery should be placed on top of the ribbon to help with future removal and replacement.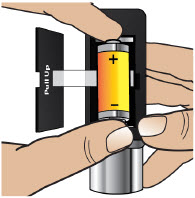 Figure GA
Figure GA
Step 17.9 Close the battery door by pushing it back into place (Figure GB). 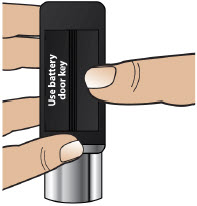 Figure GB
Figure GB
You will hear a series of beeps when the new battery is fully inserted (Figure GC).  Figure GC
Figure GC
The pump screen will wake up and show the logo screen followed by the Infusion Off screen (Figure GD).  Figure GD
Figure GDAlarms & Errors Within this section you will find: Error Codes 91 Troubleshooting 98 Alarms and Troubleshooting
Error Codes An error code will display on the screen if there is something wrong with the pump. You will hear a beep when there is an error code, and the LED next to the screen may light up. The table below gives an overview of the possible error codes that may happen, what they mean, and what to do to fix them.
If the corrective actions provided below do not fix the issue, contact Customer Support at 1-833-3ONAPGO (1-833-366-2746).

Do nottry to start or continue with your infusion when the screen displays an error. This will help avoid overdose or underdose. Blank Screen Error If a Blank Screen Error occurs, follow the steps below.  Figure GE
Figure GEWhat is happening? You will hear intermittent beeps. The screen is blank and the LED lights up (Figure GE). What does it mean? The pump has detected an error that needs to be addressed. What to do? Contact Customer Support at 1-833-3ONAPGO (1-833-366-2746). Error 2 If Error 2 occurs, follow the steps below.  Figure GF
Figure GF
 Figure GG
Figure GGWhat is happening? You will hear a continuous tone and the screen will stay on and show a notification (Figure GF) until the error is addressed. What does it mean? The pump has detected an error that needs to be addressed. What to do? -
Press the OK button (
 ) to clear the error (Figure GF). The pump will try to fix itself.
) to clear the error (Figure GF). The pump will try to fix itself.
- If the error clears, you can continue with your infusion as normal.
- If the error message remains, contact Customer Support at 1-833-3ONAPGO (1-833-366-2746). (Figure GG).
Error 3, Error 5, Error 7 If Error 3, Error 5, or Error 7 occurs, follow the steps below.  Figure GH
Figure GH
 Figure GI
Figure GIWhat is happening? You will hear intermittent beeps. The screen will show a notification (Figure GH) and the LED may light up. What does it mean? The pump has detected an error that needs to be addressed. What to do? -
Press the OK button (
 ) to clear the error (Figure GH). The pump will try to fix itself.
) to clear the error (Figure GH). The pump will try to fix itself.
- If the error clears, you can continue with your infusion as normal.
- If the error message remains, contact Customer Support at 1-833-3ONAPGO (1-833-366-2746) (Figure GI).
Error 4 If Error 4 occurs, follow the steps below.  Figure GJ
Figure GJWhat is happening? You will hear intermittent beeps and the screen will stay on and show a notification (Figure GJ) until the error is addressed. What does it mean? The pump has detected an error that needs to be addressed. What to do? Contact Customer Support at 1-833-3ONAPGO (1-833-366-2746) (Figure GJ). Error 6, Error 8, Error 9, Error 11 If Error 6, Error 8, Error 9, or Error 11 occurs, follow the steps below. *Note: Images below show Error 6 as an example.  Figure GK
Figure GK
 Figure GL
Figure GLWhat is happening? You will hear intermittent beeps and the screen will stay on and show a notification (Figure GK) until the error is addressed. What does it mean? The pump has detected an error that needs to be addressed. What to do? -
 If attached to the body, remove the infusion line.
If attached to the body, remove the infusion line.
-
Press the OK button (
 ) to clear the error (Figure GK). The pump will try to fix itself.
) to clear the error (Figure GK). The pump will try to fix itself.
- If the error clears, you can continue with your infusion as normal.
- If the error message remains, contact your healthcare provider orclinical nurse navigator (Figure GL).
Blockage If the Blockage Error occurs, follow the steps below.  Figure GM
Figure GM
 Figure GN
Figure GN
 Figure GO
Figure GO
 Figure GP
Figure GPWhat is happening? You will hear intermittent beeps and the screen will stay on and show a notification (Figure GM) until the error is addressed. What does it mean? Too much pressure has been detected in the Infusion Line. Something may be blocking the infusion flow. What to do? -
 If attached to the body, remove the infusion line site connector from your body while leaving the cannula connected to your skin (Figure GM). Press the OK button (
If attached to the body, remove the infusion line site connector from your body while leaving the cannula connected to your skin (Figure GM). Press the OK button (
 ).
).
- Straighten the infusion line out completely to remove any kinks from the line.
- Make sure the infusion line site connector end is over a sink or a towel before pressing the OK button. After the blockage is cleared, some drug may come out.
-
Press the OK button (
 ) to clear the error (Figure GN). The pump will try to fix itself.
) to clear the error (Figure GN). The pump will try to fix itself.
-
If the blockage is cleared, the infusion will continue. Reattach the infusion line to your body and press the OK button (
 ) (Figure GO).
) (Figure GO).
- If the error message remains, Contact your healthcare provider or clinical nurse navigator (Figure GP).
Warning:If something is blocking the infusion of ONAPGO, such as a kink, be sure to disconnect the infusion line before clearing the blockage alarm. If the infusion line is attached to the body when the error is cleared, you may receive a small additional amount of medicine. Low Battery If the Low Battery Alert occurs, follow the steps below.  Figure GQ
Figure GQ
 Figure GR
Figure GRWhat is happening? You will hear a beep and see a notification (Figure GQ or Figure GR) on the screen. What does it mean? The battery is getting low. What to do? During an Infusion (Figure GQ): -
Press the OK button (
 ) to clear alert (Figure GQ).
) to clear alert (Figure GQ).
- Replace the battery before the next infusion.
See Replacing the Batterysection on pages 81 to 85 for detailed instructions.
Not during an Infusion (Figure GR):
- A battery symbol will appear in the top right corner (Figure GR).
- Replace the battery before next infusion.
See Replacing the Batterysection on pages 81 to 85 for detailed instructions on how to replace the pump battery.
Replace Now (Battery Dead) If the Replace Now Alarm occurs, the battery is dead. Follow the steps below.  Figure GS
Figure GSWhat is happening? You will see a notification (Figure GS) on screen. What does it mean? The battery is dead and must be replaced right away.
Note:The pump will not work if the battery is dead.What to do? The screen will show a notification (Figure GS): - Before setting up or starting an infusion, replace the battery.
See Replacing the Batterysection on pages 81 to 85 for detailed instructions on how to replace the battery.
Note:The alarms have no intentional delays and are raised as soon as the safety system detects the issue. Note:The pump's firmware keeps the safety system under constant control, and an Error 2 alarm is raised if any issue is detected. Note:The LED is used only when it is not possible to show the error on the display. The fact that the LED is not used in other alarms does not necessarily mean that they are of a lower priority. Troubleshooting: If Your Infusion Line Did Not Prime Follow the steps below if your infusion line did not prime correctly: Wake up the pump (if needed) and then repeat the priming procedure. To do this, go to the menu and select Repeat Prime (Figure GT). When priming is complete, continue the procedure by skipping to Step 4.8.  Figure GT
Figure GT
If no liquid drops came out of the site connector end after priming two times, select Endon the Menu screen and throw away the infusion line in a sharps container. Then connect a new Infusion Line and start the priming process again beginning with Step 4.1 on page 33.
See the Disposalsection on page 73 for information on how to safely throw away ONAPGO.Symbols Used on the ONAPGO Pump

Caution:Consult accompanying documents for important safety-related information. 
CF Applied Part, refer to Technical Manual for more information. 
Caution:Consult Instructions for Use. 
Do not use in or next to a Magnetic Resonance Imaging (MRI) medical device. 
Ingress Protection (IP) rating.
Keep pump dry. If wet, wipe off with a dry cloth. The pump must not be immersed in water and must not be used while showering, swimming, or in a sauna.
Prescription device. 
Only use the pump with a CR123A battery. Technical characteristics of the Pump
Dimensions and weight Dimensions 81 × 49 × 31 mm Dimensions with holder attached 149 × 49 × 31 mm Weight 127g Technical data Power Supply CR 123A 3V lithium battery Battery life Approximately 90 infusions Display Color OLED 96×64 pixels Ingress protection IP 42 Buzzer sound pressure level 53 dBA +/- 5 dBA Data retention The value settings and counters are retained even without the battery. Maximum service life Two years from date of purchase. Infusion Data
Note: The information below describes what the pump can do, but it may not be the same as your prescription.Administrable volume Max. 20 ml Infusion flow rate range 1.0 – 8.0 mg/h (drug concentration is 98 mg/20 mL (4.9 mg/ml)) Flow rate programming increment 0.1 mg/h Shot volume 20 μl Extra dose (bolus dose) amount 0.5 - 4.0 mg. May also be set to 0 mg Bolus dose amount programming increment 0.1 mg Maximum number of extra dose (bolus doses) per day 0-5 Bolus doses per infusion programming increment 1 Infusion Time Setting 1 - 24 h Maximum infusion time programming increment 1h Minimum interval between extra doses (bolus doses) 3 h Bolus dose interval programming increment 30 minutes Bolus dose flow rate Max. 163 mL/hr Bolus dose accuracy Low dose 0.5 to 1.0 mg +/- 25%
High dose 1.5 to 4.0 mg +/- 15%Flow rate accuracy Low flow rate 1.0 to 3.5 mg/hr: +/- 15%
High flow rate 4.0 to 8.0 mg/hr: +/- 10%Maximum difference in height between pump and infusion site 50 cm Priming volume Fixed 1 ml (first delivery), 0.6 ml (second delivery) Flow rate during priming 163 ml/h Occlusion pressure levels 2.5 +/- 1.5 bar (36 +/- 22 psi) Maximum occlusion detection time (hh:mm) at a flow rate of 1mg/h: 4h 50m
at a flow rate of 8mg/h: 32mPost-occlusion bolus (ml) 0.85ml Factory Settings Infusion flow rate 1.0 mg/h Maximum infusion time 1 h Bolus dose amount 0.0 mg Minimum interval between bolus doses 3 h Maximum number of bolus doses per infusion 0 Environmental Conditions for Use Temperature +5 – +40 °C (+41 – +104°F) Relative humidity 15 – 68% Pressure 0.7 – 1.06 bar (10 – 15 psi) Environmental Conditions for Storage Temperature -25 – +70 °C (-13 – +158°F) Relative humidity Max 68% Pressure 0.5 – 1.06 bar (7 – 15 psi) Electromagnetic Compatibility
This section prescribes the correct environments for use in accordance with the declared regulations on electromagnetic compatibility.
Reference standards
The electromagnetic compatibility tests were carried out in accordance with the following standards:
- IEC 60601-2-24:2012 (medical electrical equipment)
- IEC 60601-1-2:2014 (medical electrical equipment)
Electromagnetic emissions
The manufacturer's declarations and requirements are set out below:
Emission test Conformity Indications regarding electromagnetic environment RF emissions CISPR 11 Group 1 The pump uses radio frequency only for its internal operation. As a consequence, its RF emissions are very low and would thus not be expected to cause any interference to electronic devices in the vicinity. RF emissions CISPR 11 Class B The pump can be used in all environments, including domestic environments that are directly connected to a low-voltage public mains power network. Electromagnetic Immunity
The manufacturer's declarations and requirements are set out below:
Immunity test Test level Conformity level Indications regarding electromagnetic environment. Electrostatic discharge (ESD) IEC 61000-4-2 15 kV air 8 kV contact 15 kV air 8 kV contact The pump must be used in an environment with wood, concrete or ceramic flooring. Where floors are covered with synthetic material, the relative humidity should be at least 30%. Magnetic fields 30 A/m 50 and 60 Hz 30 A/m 50 and 60 Hz Radiated immunity 80-2700 MHz AM 80% 1 KHz 10 V/m
The pump may be subject to interference in the vicinity of devices marked with the following symbol:
 WARNING! Underdose or overdose due to abnormal pump operation. If the pump is used in the vicinity of other devices, it should be kept under observation in order to verify that no alarms are shown and that the infusion proceeds normally, delivering the drug in the expected time. If in doubt, do not use the device.
WARNING! Underdose or overdose due to abnormal pump operation. If the pump is used in the vicinity of other devices, it should be kept under observation in order to verify that no alarms are shown and that the infusion proceeds normally, delivering the drug in the expected time. If in doubt, do not use the device.
IEC 61000-4-3380-390 MHz 18Hz PM 50% 27 V/m 430-470 MHz 18Hz PM 50% 28 V/m 704-787 MHz 217Hz PM 50% 9 V/m 800-960 MHz 18Hz PM 50% 28 V/m 1700-1990 MHz 217Hz PM 50% 28 V/m 2400-2570 MHz 217Hz PM 50% 28 V/m 5100-5800 MHz 217Hz PM 50% 9 V/m Recommended separation distances from radio frequency devices
Portable radio frequency communication devices (including external antennas and their cables) may interfere with the pump if they are too close to it. These include mobile and cordless phones, LAN routers and intercom systems for children.
To avoid electromagnetic interference, use the pump at a distance at least 30 cm from such devices.
Tests of Accuracy
The accuracy of pump delivery has been evaluated based on tests carried out during an infusion in accordance with the following standards:
- IEC 60601-2-24:2012 (medical electrical equipment)
Note:Pump and drug cartridge have only been tested with Cleo90 infusion set which is recommended for use. Alternative accessories and extensions may cause inaccuracies in flow rate.
The total administrable volume of the drug is a maximum of 20 mL, and the drug concentration is 98 mg/20 mL (4.9 mg/mL). The flow rate programming increment is 0.5 mg per hr. The maximum infusion programming time is in increment of one hour. The accuracy of the pump was tested for a flow rate between 1.0 mg and 8.0 mg per hour. (See the Prescribing Information for recommended dosage). The flow rate stabilizes within minutes from initiation of the infusion, remains stable and delivers accurately for the duration of infusion as described in the technical specifications provided.
Limited Warranty
The pump is covered by a manufacturer's warranty for two (2) years. After this period, the manufacturer shall not be responsible for any repairs.
The manufacturer shall not be liable for damage to persons or property after a pump lifetime of two (2) years.
Contact & Support
For help and additional information on the pump or system, contact Customer Support at 1-833-3ONAPGO (1-833-366-2746).
Manufactured for:
MDD US Operations, LLC, a
subsidiary of Supernus
Pharmaceuticals, Inc.
Rockville, MD 20850MDD US Operations, LLC is the exclusive licensee and distributor of ONAPGO in the United States and Its territories.
©2025. ONAPGO is a registered trademark of BRITUSWIP LTD.
Version 14
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 06/2025
-
Healthcare Provider
INSTRUCTIONS FOR USE
ONAPGO™ (on-AP-goh)

This Instructions for Use contains information for healthcare providers on how to program the infusion pump to administer ONAPGO (apomorphine hydrochloride).
Please see the Important Safety Information, full Prescribing Information, and Patient Informationfor ONAPGO.
MDD US Operations, LLC
Table of Contents Table of Contents 2 ONAPGO Infusion System 3 Indication for Use 3 Introduction to the Infusion System 3 Pump Warnings and Precautions 4 Pump Kit 5 Pump Diagram 5 Pump Screen Icons 7 Programming the ONAPGO Pump 8 Settings Overview 8 Step 1: Access Pump Settings 9 Step 2: Enter Password 10 Step 3: Program Pump Settings 11 Symbols Used on the ONAPGO Pump 20 Limited Warranty 21 Contact & Support 21 ONAPGO Infusion System
Indication for Use The ONAPGO Infusion Pump is for the subcutaneous infusion of ONAPGO (apomorphine hydrochloride). ONAPGO is indicated for the treatment of motor fluctuations in adults with advanced Parkinson's disease (PD).
It must be used only by individuals who have received instruction and training on its operations and on the correct, safe procedures. If patients are unable to give treatment independently, they must be given assistance and supervision.
Only the accessories supplied by CANÈ S.p.A. (plastic cartridge holder and supplies provided in the infusion pump kit) may be used in conjunction with this pump. The pump must be programmed by an adequately trained healthcare provider and/or a Supernus Pharmaceuticals/MDD US Operations Clinical Nurse Navigator (clinical nurse navigator).
Introduction to the Infusion System Below are the common terms used throughout this Instructions for Use.
- Pump:controls the rate and amount of ONAPGO delivered to the body. It is programmed with the patient's prescription and displays the status of medicine delivery.
- Priming:refers to the task of removing all air from the Infusion Line (fluid pathway) before the infusion line is connected to the body.
- Flow Rate:the constant flow of ONAPGO given to the body over an extended period of time. The flow rate is expressed as milligrams per hour (mg/hr).
- Infusion Time:the total time that ONAPGO is infused per day.
- Extra Dose (+ Dose):a boost of ONAPGO (bolus dose) given over a short period of time in addition to the prescribed flow rate.
- Extra Dose (+ Dose) Lock:the amount of time before another extra dose can be given.
- Pump Settings:the patient's prescribed Flow Rate, Maximum Infusing Time Per Day, +Dose Quantity, +Dose Volume, and +Dose Lock. These settings can only be changed by a Healthcare Provider and/or Supernus Clinical Nurse Navigator.
Pump Warnings and Precautions  WARNINGS:
WARNINGS:
- Do notgive a patient a pump without checking the prescription. Always check that the pump Settings match the patient's prescription before giving the pump to a patient.
- Do notstart an infusion without confirming the patient's prescription. If the pump settings do not match the prescription, do not allow the patient to use the pump. Reprogram the pump to match the prescription.
- Do notgive a patient more than 98 mg of ONAPGO over a 24-hour period.
- Do notdisclose the pump's security codes or other information to the patient. It would allow them access to the programming functions.
- Use ONAPGO drug cartridges with the ONAPGO pump only.
 PRECAUTIONS:
PRECAUTIONS:
- Do notuse sharp objects to press the pump buttons. Only use your fingertips.
- Do notuse a damaged pump. If you have any concerns about the correct functioning of the pump, and/or how to respond to an error contact your customer support representative.
Pump Kit (Figure A) 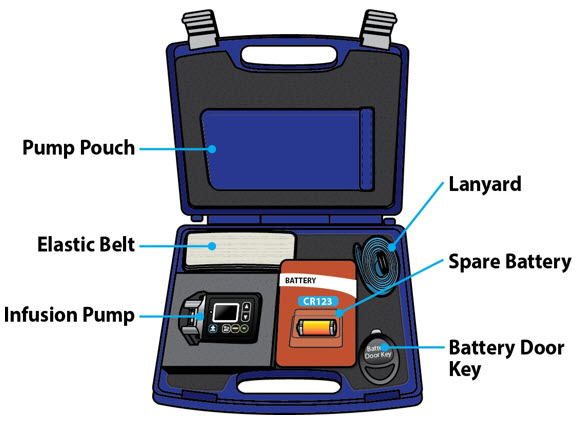
Pump Diagram (Figures B, C, and D) 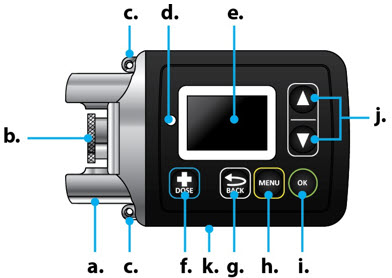 Figure B
Figure B
Figure D Figure C
Figure CAll buttons will respond with a single press. If the screen is dark, press the UP ARROW (
 ) to wake up the screen.
) to wake up the screen.
Item Pump Feature Description a Cartridge Connection Area where assembled glass drug cartridge and plastic cartridge holder is attached to the pump. b Pump Pusher Pushes ONAPGO from the glass drug cartridge into the Infusion Line. c Neck Lanyard Clips Slots to clip on the lanyard. d LED Lights up when an error cannot be displayed on the screen. e Screen Where messages and menus appear. f 
+DOSE button gives an extra dose (if prescribed). g 
BACK button allows you to return to a previous screen, menu, or cancel an action. h 
MENU button allows you to access the menus. i 
OK button allows you to confirm menu selections or go on to the next value. j 
UP and DOWN ARROW buttons allow you to scroll through menus. k Battery Door Door to access the battery. Pump Screen Icons The following table shows Icons that appear on the Pump screen.
Icon Name Description 
Back Arrow The back arrow is shown when checking the pump settings. This icon is used to show that pressing the BACK button will return to a previous screen (Item 'g' in Figure B). 
Caution Caution is shown when an error or alarm is occurring. 
Check Mark A check mark is shown when the Pump successfully completes a task. 
Droplet A blinking droplet is shown on the Infusing Screen when an infusion is on. 
Dead Battery The dead battery is shown when the Pump battery is dead and must be replaced. 
Low Battery The low battery is shown when the pump battery is low and should be replaced before the next infusion. 
OK Arrow The OK arow is shown when checking the pump settings. This icon is used to show that pressing the OK button will confirm a selection or go on to the next value (Item 'i' in Figure B). 
Padlock Padlock is shown when the +Dose (extra dose) is not available. 

Pause Pause is shown when the infusion is paused. 
+Dose
(Extra Dose)A +symbol refers to the extra dose ( +Dose) function. Programming the ONAPGO Pump
The pump cannot be programmed while infusing.
The pump settings should be programmed according to the patient's prescription before they receive the pump.
Settings Overview When programming the pump, a flashing value is one that you may edit. The table below describes the default pump settings and range of values to which the pump can be programmed. See the Prescribing Information for the recommended dosage regimen, including the recommended flow rate (i.e., 1 mg/hr to 6 mg/hr), infusion time (i.e., generally over 16 waking hours), and extra doses (i.e., no more than 3 per day).
Icon Default Pump Setting Range of Values Increments Refer To Time Factory setting 12:00 AM - 11:59 PM N/A Step 3.1
(page 11)Date Factory setting mm/dd/yy N/A Step 3.2
(page 12)Flow Rate 1 mg/hr 1 - 8 mg/hr 0.5 mg/hr Step 3.3
(page 13)Infusion Time 1 hr 1 - 24 hr 1 hr Step 3.4
(page 14)+Doses/Day
(Extra Doses/Day)0
(No Bolus)0 - 5 1 Step 3.5
(page 15)+Dose 0 mg 0.5 - 4 mg 0.5 mg Step 3.6
(page 16)+Dose Lock 3 hr 0 - 24 hr 30 min Step 3.7
(page 17)Step 1: Access Pump Settings Step 1.1
If the pump screen is asleep, press the UP ARROW ( ) to wake it up (Figure E).
) to wake it up (Figure E).
Note:Do this anytime you need to wake up the pump. Figure E
Figure E
When the pump wakes up, the screen will say Infusion Off (Figure F). 
Figure F
Step 1.2
Press and hold the OK button ( ). While continuing to hold, press and hold the UP ARROW at the same time (Figure G), until a password input screen appears (Figure H).
). While continuing to hold, press and hold the UP ARROW at the same time (Figure G), until a password input screen appears (Figure H).
You will hear a series of beeps when holding both the UP ARROW ( ) and OK button (
) and OK button (
 ).
).
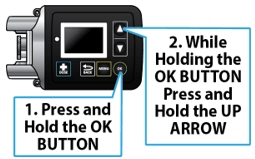
Figure G
Keep holding both buttons until the password screen appears.
You will then see the password screen (Figure H). Figure H
Figure HStep 2: Enter Password Important Information:
 Do notdisclose the pump's security codes or other information to the patient. It would allow them access to the programming functions.
Do notdisclose the pump's security codes or other information to the patient. It would allow them access to the programming functions.
Step 2.1
Locate the 4-digit password on the back of the pump (Figure I).
The password is the 4 numbers following 'AJ'. In the example provided (Figure I), the 4-digit password would be 0001.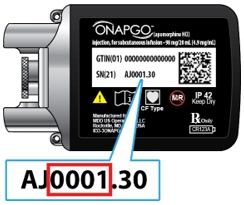
HCP password on back of pump
Figure I
Step 2.2
Use the UP AND DOWN ARROWS ( ) to change the values on the Password screen (Figure J). Flashing values indicate that they are currently being edited. When the selected number matches the pump password, press the OK button (
) to change the values on the Password screen (Figure J). Flashing values indicate that they are currently being edited. When the selected number matches the pump password, press the OK button (
 ) to proceed to the next number. Repeat this process for all 4 numbers.
) to proceed to the next number. Repeat this process for all 4 numbers.
 Figure J
Figure J
When the correct password has been entered, press the OK button (
 ) to proceed to the Time screen (Figure K).
) to proceed to the Time screen (Figure K).
Note:If incorrect password is entered, the pump will go to the Infusion Off screen. Repeat steps 2.1 and 2.2 (above) with the correct password. Figure K
Figure KStep 3: Program Pump Settings If this is the first time the pump will be programmed, the existing values will reflect the default factory settings (Page 8).
For any subsequent programming, the values will reflect the previously programmed patient prescription.
The settings will need to be changed to match the time, date, and patient's prescription.
Step 3.1
Use the UP and DOWN ARROWS ( ) to change the hour. As the hour is adjusted, the AM and PM will also change. When the hour and AM/PM are correct, press the OK button (
) to change the hour. As the hour is adjusted, the AM and PM will also change. When the hour and AM/PM are correct, press the OK button (
 ) to change the minutes (Figure L). Use the UP and DOWN ARROWS (
) to change the minutes (Figure L). Use the UP and DOWN ARROWS (
 ) to change the minutes.
) to change the minutes.
 Figure L
Figure L
When the Time is correct, press the OK button (
 ) to proceed to the Date screen (Figure M) Step 3.2.
) to proceed to the Date screen (Figure M) Step 3.2.
 Figure M
Figure M
Step 3.2
Use the UP and DOWN ARROWS ( ) to change the month and press the OK button (
) to change the month and press the OK button (
 ) to continue to change the day and year (Figure N).
) to continue to change the day and year (Figure N).
 Figure N
Figure N
When the date is correct, press the OK button (
 ) to proceed to the Flow Rate screen (Figure O) Step 3.3.
) to proceed to the Flow Rate screen (Figure O) Step 3.3.
 Figure O
Figure O
Step 3.3
Use the UP and DOWN ARROWS ( ) to change the flow rate (Figure P).
) to change the flow rate (Figure P).
 Figure P
Figure P
When the flow rate is correct, press the OK button (
 ) to proceed to the Infusion Time screen (Figure Q) Step 3.4.
) to proceed to the Infusion Time screen (Figure Q) Step 3.4.
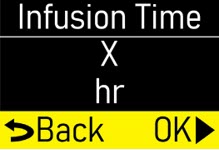 Figure Q
Figure Q
Note:If you are prescribing above the recommended flow rate, a Verify screen will appear (Figure R).
Press the OK button ( ) to proceed or press the BACK button (
) to proceed or press the BACK button (
 ) to edit the flow rate.
) to edit the flow rate.
 Figure R
Figure R
Step 3.4
Use the UP and DOWN ARROWS ( ) to change the Infusion Time (length of time that infusion will run) (Figure S).
) to change the Infusion Time (length of time that infusion will run) (Figure S).
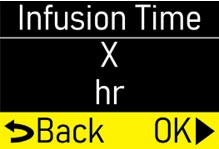 Figure S
Figure S
When the infusion time is correct, press the OK button (
 ) to proceed to the + Doses/Day (extra doses/day) screen (Figure T) Step 3.5.
) to proceed to the + Doses/Day (extra doses/day) screen (Figure T) Step 3.5.
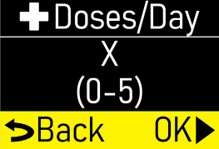 Figure T
Figure T
Note:If you are prescribing above the recommended infusion time, a Verify screen will appear (Figure U).
Press the OK button ( ) to proceed or press the BACK button (
) to proceed or press the BACK button (
 ) to edit the infusion time.
) to edit the infusion time.
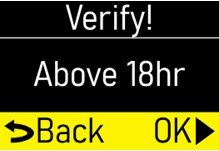 Figure U
Figure U
Step 3.5
Use the UP and DOWN ARROWS ( ) to change the number of allowable
+Doses (extra doses) that can be delivered per Day (Figure V).
) to change the number of allowable
+Doses (extra doses) that can be delivered per Day (Figure V).
Note:If no +dose (extra dose) is prescribed, enter '0' for the number of +Doses/Day (extra doses/ day) and press the OK button ( ) to proceed to the
+Dose screen (Figure W) Step 3.6. If no
+Doses/Day (extra doses/day) are prescribed, the subsequent
+Dose and
+Dose Lock screen values cannot be changed.
) to proceed to the
+Dose screen (Figure W) Step 3.6. If no
+Doses/Day (extra doses/day) are prescribed, the subsequent
+Dose and
+Dose Lock screen values cannot be changed.
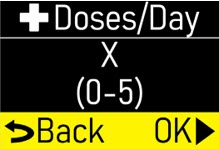 Figure V
Figure V
When the +Doses/Day (extra doses/ day) number is correct, press the OK button (
 ) to proceed to the
+Dose screen (Figure W) Step 3.6.
) to proceed to the
+Dose screen (Figure W) Step 3.6.
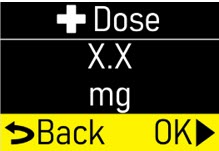 Figure W
Figure W
Note:If you are prescribing above the recommended +Doses/Day, a Verify screen will appear (Figure X).
Press the OK button ( ) to proceed or press the BACK button (
) to proceed or press the BACK button (
 ) to edit the
+Doses/Day (extra doses/day).
) to edit the
+Doses/Day (extra doses/day).
 Figure X
Figure X
Step 3.6
Use the UP and DOWN ARROWS ( ) to change the
+Dose (Extra Dose) value (mg) (Figure Y).
) to change the
+Dose (Extra Dose) value (mg) (Figure Y).
Note:If no extra doses are prescribed, the +Dose value cannot be changed. Press the OK button ( ) to proceed to the
+Dose Lock screen (Figure Z) Step 3.7.
) to proceed to the
+Dose Lock screen (Figure Z) Step 3.7.
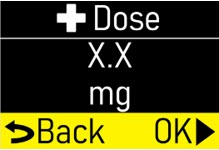 Figure Y
Figure Y
When the +Dose (extra dose) value is correct, press the OK button (
 ) to proceed to the
+Dose Lock screen (Figure Z) Step 3.7.
) to proceed to the
+Dose Lock screen (Figure Z) Step 3.7.
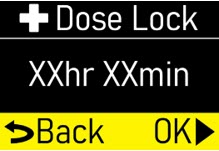 Figure Z
Figure Z
Note:If you are prescribing above the recommended extra doses, a Verify screen will appear (Figure AA). Press the OK button (
 ) to proceed or press the BACK button (
) to proceed or press the BACK button (
 ) to edit the
+Dose (extra dose).
) to edit the
+Dose (extra dose).
 Figure AA
Figure AA
Step 3.7
Use the UP and DOWN ARROWS ( ) to change the + Dose Lock (extra dose lock) time (time patient must wait before another extra dose) (Figure AB).
) to change the + Dose Lock (extra dose lock) time (time patient must wait before another extra dose) (Figure AB).
Note:If no extra doses are prescribed, the + Dose (extra dose) lock time cannot be changed. Press the OK button ( ) to proceed to the Verify Rx screen (Figure AC) Step 3.8.
) to proceed to the Verify Rx screen (Figure AC) Step 3.8.
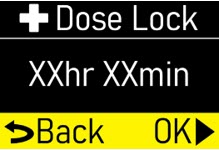 Figure AB
Figure AB
When the + Dose (extra dose) lock time is correct, press the OK button (
 ) to proceed to the Verify Rx screen (Figure AC) Step 3.8.
) to proceed to the Verify Rx screen (Figure AC) Step 3.8.
 Figure AC
Figure AC
Step 3.8
When the Verify Rx screen appears (Figure AD), press the DOWN ARROW ( ) to confirm that each pump setting programmed matches the patient's prescription.
) to confirm that each pump setting programmed matches the patient's prescription.
 Figure AD
Figure AD
Continue pressing the DOWN ARROW (
 ) until the Back/OK row is highlighted (Figure AE).
) until the Back/OK row is highlighted (Figure AE).
 Figure AE
Figure AE
Step 3.9
If the programmed pump settings match the patient's prescription, press the OK button ( ) to save the pump settings (Figure AF).
) to save the pump settings (Figure AF).
Note:If any of the programmed pump settings do not match the patient's prescription, use the BACK button ( ) to go back to the setting and adjust accordingly.
) to go back to the setting and adjust accordingly.

Figure AFWhen the pump is done saving, the Infusion Off screen will appear (Figure AG).
Note:If the OK button is not pressed and the pump screen falls asleep (goes dark), the programmed pump settings will still be saved. Figure AG
Figure AGSymbols Used on the ONAPGO Pump

Caution:Consult accompanying documents for important safety-related information. 
CF Applied Part, refer to Technical Manual for more information. 
Caution:Consult Instructions for Use. 
Do not use in or next to a Magnetic Resonance Imaging (MRI) medical device. 
Ingress Protection (IP) rating.
Keep pump dry. If wet, wipe off with a dry cloth. The pump must not be immersed in water and must not be used while showering, swimming, or in a sauna.
Prescription device. 
The pump is to only be used with a CR123A battery. Note:The symbols displayed on the product label are reduced and simplified compared to the specifications in the reference standard CENELEC EN50419.
Limited Warranty
The pump is covered by a manufacturer's warranty for two (2) years. After this period, the manufacturer shall not be responsible for any repairs.
The manufacturer shall not be liable for damage to persons or property after a pump lifetime of two (2) years.
Contact & Support
For help and additional information on the pump or system, contact Customer Support at 1-833-3ONAPGO (1-833-366-2746).
Manufactured for:
MDD US Operations, LLC, a
subsidiary of Supernus Pharmaceuticals, Inc.
Rockville, MD 20850MDD US Operations, LLC is the exclusive licensee and distributor of ONAPGO in the United States and Its territories.
©2025. ONAPGO is a registered trademark of BRITUSWIP LTD.
Version 8
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 06/2025
-
PRINCIPAL DISPLAY PANEL - 20 mL Cartridge Carton
ONAPGO™
(apomorphine HCl)
injection, for subcutaneous useNDC: 27505-006-05
Rx Only98 mg/20 mL (4.9 mg/mL)
Single-dose cartridge.
Discard unused portion.Package contains 5 x 20 mL cartridges

-
INGREDIENTS AND APPEARANCE
ONAPGO
apomorphine hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 27505-006 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APOMORPHINE HYDROCHLORIDE (UNII: F39049Y068) (APOMORPHINE - UNII:N21FAR7B4S) APOMORPHINE HYDROCHLORIDE 4.9 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM METABISULFITE (UNII: 4VON5FNS3C) 0.5 mg in 1 mL HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 27505-006-05 5 in 1 CARTON 02/04/2025 1 NDC: 27505-006-01 20 mL in 1 CARTRIDGE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214056 02/04/2025 Labeler - MDD US Operations, LLC, a subsidiary of Supernus Pharmaceuticals, Inc (087875626)
Trademark Results [ONAPGO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ONAPGO 98402022 not registered Live/Pending |
BRITUSWIP LTD 2024-02-12 |
 ONAPGO 90104134 not registered Live/Pending |
BRITUSWIP LTD 2020-08-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.